当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical water oxidation by a copper complex with an N4-donor ligand under neutral conditions
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1cy01183a Junqi Lin 1 , Xin Chen 1 , Nini Wang 1 , Shanshan Liu 1 , Zhijun Ruan 1 , Yanmei Chen 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1cy01183a Junqi Lin 1 , Xin Chen 1 , Nini Wang 1 , Shanshan Liu 1 , Zhijun Ruan 1 , Yanmei Chen 1
Affiliation
|
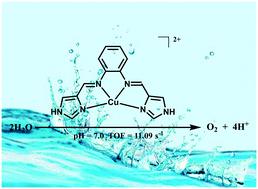
Herein, electrochemical water oxidation catalyzed by a copper(II) complex [CuII(H2L)](NO3)2 with the redox-active salophen-like N4-donor ligand N,N′-bis-(1H-imidazol-4-yl)methylidene-o-phenylenediamine is demonstrated. Oxygen evolution with a high turnover frequency of 11.09 s−1 and a low onset overpotential of only 580 mV is achieved in neutral phosphate buffer solution, and [CuII(H2L)]2+ is confirmed as an efficient molecular water oxidation catalyst with long-term stability. The results of electrochemical tests provide evidence that the Cu center undergoes a water nucleophilic attack process and participates in the catalytic cycle. The subsequent one-step proton-coupled electron transfer (PCET) process of the Cu center and the two-step PCET process of the ligand are both critical for efficient water oxidation. This work indicates that the ligand assisted catalytic cycle is a favorable method for the accumulation of intermediate species that account for electrochemical water oxidation.
中文翻译:

中性条件下铜配合物与 N4 供体配体的电化学水氧化
在此,由铜 ( II ) 配合物 [Cu II (H 2 L)](NO 3 ) 2催化的电化学水氧化与具有氧化还原活性的类卤苯N 4 -供体配体N , N '-bis-(1 H对-咪唑-4-基)亚甲基-邻苯二胺进行说明。在中性磷酸盐缓冲溶液和 [Cu II (H 2 L)] 2+ 中实现了具有 11.09 s -1的高周转频率和仅 580 mV 的低起始过电位的氧气释放被证实是一种具有长期稳定性的高效分子水氧化催化剂。电化学测试结果证明,Cu 中心经历了水亲核攻击过程并参与了催化循环。随后铜中心的一步质子耦合电子转移 (PCET) 过程和配体的两步 PCET 过程都是有效水氧化的关键。这项工作表明,配体辅助催化循环是一种有利的方法,可用于积累导致电化学水氧化的中间物种。
更新日期:2021-09-08
中文翻译:

中性条件下铜配合物与 N4 供体配体的电化学水氧化
在此,由铜 ( II ) 配合物 [Cu II (H 2 L)](NO 3 ) 2催化的电化学水氧化与具有氧化还原活性的类卤苯N 4 -供体配体N , N '-bis-(1 H对-咪唑-4-基)亚甲基-邻苯二胺进行说明。在中性磷酸盐缓冲溶液和 [Cu II (H 2 L)] 2+ 中实现了具有 11.09 s -1的高周转频率和仅 580 mV 的低起始过电位的氧气释放被证实是一种具有长期稳定性的高效分子水氧化催化剂。电化学测试结果证明,Cu 中心经历了水亲核攻击过程并参与了催化循环。随后铜中心的一步质子耦合电子转移 (PCET) 过程和配体的两步 PCET 过程都是有效水氧化的关键。这项工作表明,配体辅助催化循环是一种有利的方法,可用于积累导致电化学水氧化的中间物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号