Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.jmgm.2021.108027 Min Zhou 1 , Huilin Wen 1 , Huimin Lei 2 , Tao Zhang 1
|
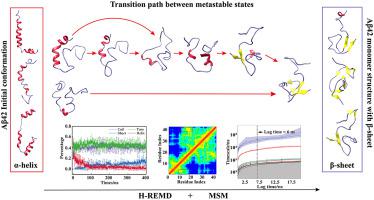
Aβ42 peptides can form helix and sheet structure under different conditions. The conformational conversion is closely associated with Aβ peptides aggregation and their neurotoxicity. But the transition from helix to sheet is not be clearly understood. In this study we performed microsecond timescale MD simulations of Aβ42 peptide to investigate the conformation transition from α-helix to β-sheet. Markov state model (MSM) was built to facilitate identification of crucial intermediate states and possible transition pathway. Based on the analysis, we found that the region Y10-A21 in the middle of Aβ42 peptide plays an initial role in this transition. MSM model revealed that the collapse of helical structure in this region might trigger the formation of sheet structure. Moreover, we further simulated the aggregation of Aβ42 peptides with different conformations. We found that the Aβ42 peptides forming sheet structure have higher aggregation potential compared with peptides with helix structure. These results demonstrate that we can prevent the aggregation of Aβ42 peptides by stabilizing the helix structure in the region of Y10-A21. In addition, this study provides new insight into better understanding the conformational transition and aggregation of Aβ42 peptides.
中文翻译:

Aβ42肽从螺旋到片层构象转变的分子动力学研究
Aβ42 肽在不同条件下可以形成螺旋和折叠结构。构象转换与 Aβ 肽的聚集及其神经毒性密切相关。但是从螺旋到片的过渡尚不清楚。在这项研究中,我们对 Aβ42 肽进行了微秒时间尺度 MD 模拟,以研究从 α-螺旋到 β-折叠的构象转变。建立马尔可夫状态模型 (MSM) 以促进关键中间状态和可能的过渡路径的识别。根据分析,我们发现 Aβ42 肽中间的 Y10-A21 区域在这种转变中起初始作用。MSM 模型显示该区域螺旋结构的坍塌可能引发片状结构的形成。而且,我们进一步模拟了不同构象的 Aβ42 肽的聚集。我们发现形成片状结构的 Aβ42 肽与具有螺旋结构的肽相比具有更高的聚集潜力。这些结果表明我们可以通过稳定 Y10-A21 区域的螺旋结构来防止 Aβ42 肽的聚集。此外,这项研究为更好地理解 Aβ42 肽的构象转变和聚集提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号