当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Heat Capacity of Non-Functional and Functional Ionic Liquids during the Absorption of SO2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.iecr.1c02327 Bingru Wang 1 , Long Lin 1 , Shuhang Ren 1 , Weize Wu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.iecr.1c02327 Bingru Wang 1 , Long Lin 1 , Shuhang Ren 1 , Weize Wu 1
Affiliation
|
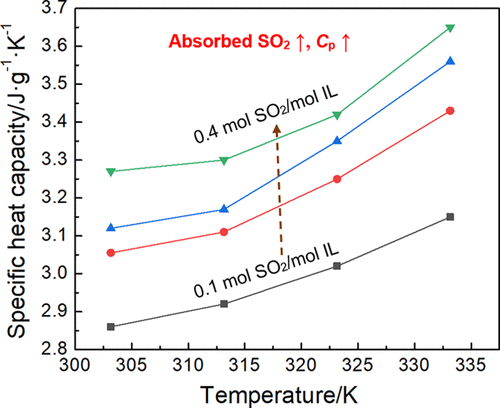
Ionic liquids (ILs), a new type of absorbents, are widely used to absorb sulfur dioxide (SO2) in flue gas. However, the lack of data on the physical properties of ILs has severely hampered their industrial applications. Unlike previous reports on the specific heat capacity of pure ILs, this work not only focuses on measuring the specific heat capacity of pure ILs but also provides the specific heat capacity of IL and SO2 mixtures during the absorption process. First, we measured the specific heat capacities of six ILs including functional ILs, 1,1,3,3-tetramethylguanidinium lactate ([TMG][Lac]), [TMG][suberate], [TMG][dodecanedioate], and 1-butyl-3-methylimidazolium acetate ([Bmim][Ac]), and non-functional ILs, [TMG][tetrafluoroborate] and [Bmim][tetrafluoroborate], at 303.15–333.15 K. We found that the specific heat capacities of the ILs with or without absorbed SO2 increase with temperature. The specific heat capacity of ILs increases with the increase of the amount of absorbed SO2. Then, we investigated the effect of the length of anionic alkyl side chains on the specific heat capacity of SO2 absorption and found that the specific heat capacity of ILs with shorter alkyl side chains was smaller than that of ILs with longer alkyl side chains. We simulated the absorption process using Gaussian software and found that the specific heat capacities of ILs and SO2 systems were mainly caused by the vibration of molecules.
中文翻译:

吸收 SO2 过程中非功能性和功能性离子液体的比热容
离子液体(ILs)是一种新型的吸收剂,广泛用于吸收烟气中的二氧化硫(SO 2)。然而,缺乏关于离子液体物理特性的数据严重阻碍了它们的工业应用。与之前关于纯 ILs 比热容的报告不同,这项工作不仅侧重于测量纯 ILs 的比热容,还提供了 IL 和 SO 2的比热容吸收过程中的混合物。首先,我们测量了六种离子液体的比热容,包括功能性离子液体、1,1,3,3-四甲基胍乳酸盐 ([TMG][Lac])、[TMG][辛二酸盐]、[TMG][十二烷二酸盐]和 1 -丁基-3-甲基咪唑鎓醋酸盐 ([Bmim][Ac]) 和非功能性离子液体 [TMG][四氟硼酸盐] 和 [Bmim][四氟硼酸盐],在 303.15–333.15 K。我们发现吸收或不吸收SO 2的离子液体随温度增加。ILs的比热容随着吸收SO 2量的增加而增加。然后,我们研究了阴离子烷基侧链的长度对 SO 2比热容的影响。吸收并发现具有较短烷基侧链的 ILs 的比热容小于具有较长烷基侧链的 ILs。我们使用高斯软件模拟了吸收过程,发现ILs 和SO 2系统的比热容主要是由分子振动引起的。
更新日期:2021-09-22
中文翻译:

吸收 SO2 过程中非功能性和功能性离子液体的比热容
离子液体(ILs)是一种新型的吸收剂,广泛用于吸收烟气中的二氧化硫(SO 2)。然而,缺乏关于离子液体物理特性的数据严重阻碍了它们的工业应用。与之前关于纯 ILs 比热容的报告不同,这项工作不仅侧重于测量纯 ILs 的比热容,还提供了 IL 和 SO 2的比热容吸收过程中的混合物。首先,我们测量了六种离子液体的比热容,包括功能性离子液体、1,1,3,3-四甲基胍乳酸盐 ([TMG][Lac])、[TMG][辛二酸盐]、[TMG][十二烷二酸盐]和 1 -丁基-3-甲基咪唑鎓醋酸盐 ([Bmim][Ac]) 和非功能性离子液体 [TMG][四氟硼酸盐] 和 [Bmim][四氟硼酸盐],在 303.15–333.15 K。我们发现吸收或不吸收SO 2的离子液体随温度增加。ILs的比热容随着吸收SO 2量的增加而增加。然后,我们研究了阴离子烷基侧链的长度对 SO 2比热容的影响。吸收并发现具有较短烷基侧链的 ILs 的比热容小于具有较长烷基侧链的 ILs。我们使用高斯软件模拟了吸收过程,发现ILs 和SO 2系统的比热容主要是由分子振动引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号