当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrostatic Drivers of GPx4 Interactions with Membrane, Lipids, and DNA
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.biochem.1c00492 Courtney L Labrecque 1 , Brian Fuglestad 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.biochem.1c00492 Courtney L Labrecque 1 , Brian Fuglestad 1, 2
Affiliation
|
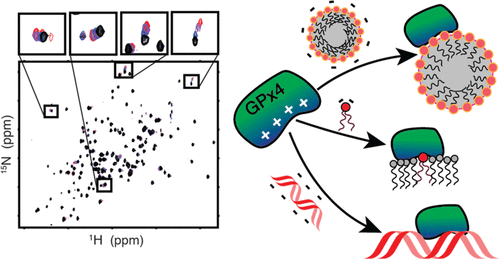
Glutathione peroxidase 4 (GPx4) serves as the only enzyme that protects membranes through the reduction of lipid hydroperoxides, preventing membrane oxidative damage and cell death through ferroptosis. Recently, GPx4 has gained attention as a therapeutic target for cancer through inhibition and as a target for inflammatory diseases through activation. In addition, GPx4 isoforms perform several distinct moonlighting functions including cysteine cross-linking of protamines during sperm cell chromatin remodeling, a function for which molecular and structural details are undefined. Despite the importance in biology, disease, and potential for drug development, little is known about GPx4 functional interactions at high resolution. This study presents the first NMR assignments of GPx4, and the electrostatic interaction of GPx4 with the membrane is characterized. Mutagenesis reveals the cationic patch residues that are key to membrane binding and stabilization. The cationic patch is observed to be important in binding headgroups of highly anionic cardiolipin. A novel lipid binding site is observed adjacent to the catalytic site and may enable protection of lipid-headgroups from oxidative damage. Arachidonic acid is also found to engage with GPx4, while cholesterol did not display any interaction. The cationic patch residues were also found to enable DNA binding, the first observation of this interaction. Electrostatic DNA binding explains a mechanism for the nuclear isoform of GPx4 to target DNA-bound protamines and to potentially reduce oxidatively damaged DNA. Together, these results highlight the importance of electrostatics in the function of GPx4 and illuminate how the multifunctional enzyme is able to fill multiple biological roles.
中文翻译:

GPx4 与膜、脂质和 DNA 相互作用的静电驱动因素
谷胱甘肽过氧化物酶 4 (GPx4) 是唯一一种通过减少脂质氢过氧化物来保护膜的酶,防止膜氧化损伤和细胞因铁死亡而死亡。最近,GPx4 通过抑制作为癌症的治疗靶点和通过激活作为炎症性疾病的靶点而受到关注。此外,GPx4 亚型执行几种不同的临时功能,包括精细胞染色质重塑过程中鱼精蛋白的半胱氨酸交联,该功能的分子和结构细节尚未确定。尽管在生物学、疾病和药物开发潜力方面具有重要意义,但人们对高分辨率的 GPx4 功能相互作用知之甚少。这项研究展示了 GPx4 的第一个 NMR 分配,并且表征了GPx4与膜的静电相互作用。诱变揭示了对膜结合和稳定至关重要的阳离子贴片残基。观察到阳离子贴剂在结合高度阴离子心磷脂的头部基团中很重要。在催化位点附近观察到一个新的脂质结合位点,可以保护脂质头基免受氧化损伤。还发现花生四烯酸与 GPx4 结合,而胆固醇没有显示任何相互作用。还发现阳离子贴片残基能够与 DNA 结合,这是对这种相互作用的首次观察。静电 DNA 结合解释了 GPx4 的核同工型靶向 DNA 结合的鱼精蛋白并可能减少氧化损伤的 DNA 的机制。一起,
更新日期:2021-09-21
中文翻译:

GPx4 与膜、脂质和 DNA 相互作用的静电驱动因素
谷胱甘肽过氧化物酶 4 (GPx4) 是唯一一种通过减少脂质氢过氧化物来保护膜的酶,防止膜氧化损伤和细胞因铁死亡而死亡。最近,GPx4 通过抑制作为癌症的治疗靶点和通过激活作为炎症性疾病的靶点而受到关注。此外,GPx4 亚型执行几种不同的临时功能,包括精细胞染色质重塑过程中鱼精蛋白的半胱氨酸交联,该功能的分子和结构细节尚未确定。尽管在生物学、疾病和药物开发潜力方面具有重要意义,但人们对高分辨率的 GPx4 功能相互作用知之甚少。这项研究展示了 GPx4 的第一个 NMR 分配,并且表征了GPx4与膜的静电相互作用。诱变揭示了对膜结合和稳定至关重要的阳离子贴片残基。观察到阳离子贴剂在结合高度阴离子心磷脂的头部基团中很重要。在催化位点附近观察到一个新的脂质结合位点,可以保护脂质头基免受氧化损伤。还发现花生四烯酸与 GPx4 结合,而胆固醇没有显示任何相互作用。还发现阳离子贴片残基能够与 DNA 结合,这是对这种相互作用的首次观察。静电 DNA 结合解释了 GPx4 的核同工型靶向 DNA 结合的鱼精蛋白并可能减少氧化损伤的 DNA 的机制。一起,



























 京公网安备 11010802027423号
京公网安备 11010802027423号