Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Osmotic Transport at the Aqueous Graphene and hBN Interfaces: Scaling Laws from a Unified, First-Principles Description
ACS Nano ( IF 15.8 ) Pub Date : 2021-09-07 , DOI: 10.1021/acsnano.1c05931 Laurent Joly 1, 2 , Robert H Meißner 3, 4 , Marcella Iannuzzi 5 , Gabriele Tocci 5
ACS Nano ( IF 15.8 ) Pub Date : 2021-09-07 , DOI: 10.1021/acsnano.1c05931 Laurent Joly 1, 2 , Robert H Meißner 3, 4 , Marcella Iannuzzi 5 , Gabriele Tocci 5
Affiliation
|
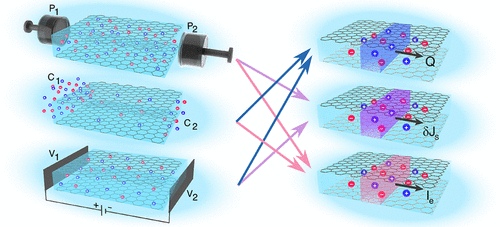
Osmotic transport in nanoconfined aqueous electrolytes provides alternative venues for water desalination and “blue energy” harvesting. The osmotic response of nanofluidic systems is controlled by the interfacial structure of water and electrolyte solutions in the so-called electrical double layer (EDL), but a molecular-level picture of the EDL is to a large extent still lacking. Particularly, the role of the electronic structure has not been considered in the description of electrolyte/surface interactions. Here, we report enhanced sampling simulations based on ab initio molecular dynamics, aiming at unravelling the free energy of prototypical ions adsorbed at the aqueous graphene and hBN interfaces, and its consequences on nanofluidic osmotic transport. Specifically, we predicted the zeta potential, the diffusio-osmotic mobility, and the diffusio-osmotic conductivity for a wide range of salt concentrations from the ab initio water and ion spatial distributions through an analytical framework based on Stokes equation and a modified Poisson–Boltzmann equation. We observed concentration-dependent scaling laws, together with dramatic differences in osmotic transport between the two interfaces, including diffusio-osmotic flow and current reversal on hBN but not on graphene. We could rationalize the results for the three osmotic responses with a simple model based on characteristic length scales for ion and water adsorption at the surface, which are quite different on graphene and on hBN. Our work provides fundamental insights into the structure and osmotic transport of aqueous electrolytes on 2D materials and explores alternative pathways for efficient water desalination and osmotic energy conversion.
中文翻译:

水性石墨烯和 hBN 界面的渗透传输:来自统一的第一性原理描述的缩放定律
纳米限界水性电解质中的渗透传输为海水淡化和“蓝色能量”收集提供了替代场所。纳米流体系统的渗透响应由所谓的双电层 (EDL) 中水和电解质溶液的界面结构控制,但在很大程度上仍缺乏 EDL 的分子级图片。特别是,在电解质/表面相互作用的描述中没有考虑电子结构的作用。在这里,我们报告了基于ab initio 的增强采样模拟分子动力学,旨在解开吸附在水性石墨烯和 hBN 界面上的原型离子的自由能,及其对纳米流体渗透传输的影响。具体来说,我们从ab initio预测了各种盐浓度的 zeta 电位、扩散渗透迁移率和扩散渗透电导率。通过基于 Stokes 方程和修正的 Poisson-Boltzmann 方程的分析框架来分析水和离子的空间分布。我们观察到浓度依赖性标度定律,以及两个界面之间渗透传输的显着差异,包括 hBN 上的扩散渗透流和电流反转,但在石墨烯上则不然。我们可以使用基于表面离子和水吸附特征长度尺度的简单模型来合理化三种渗透响应的结果,这在石墨烯和 hBN 上有很大不同。我们的工作提供了对二维材料上水性电解质的结构和渗透传输的基本见解,并探索了有效水淡化和渗透能转换的替代途径。
更新日期:2021-09-28
中文翻译:

水性石墨烯和 hBN 界面的渗透传输:来自统一的第一性原理描述的缩放定律
纳米限界水性电解质中的渗透传输为海水淡化和“蓝色能量”收集提供了替代场所。纳米流体系统的渗透响应由所谓的双电层 (EDL) 中水和电解质溶液的界面结构控制,但在很大程度上仍缺乏 EDL 的分子级图片。特别是,在电解质/表面相互作用的描述中没有考虑电子结构的作用。在这里,我们报告了基于ab initio 的增强采样模拟分子动力学,旨在解开吸附在水性石墨烯和 hBN 界面上的原型离子的自由能,及其对纳米流体渗透传输的影响。具体来说,我们从ab initio预测了各种盐浓度的 zeta 电位、扩散渗透迁移率和扩散渗透电导率。通过基于 Stokes 方程和修正的 Poisson-Boltzmann 方程的分析框架来分析水和离子的空间分布。我们观察到浓度依赖性标度定律,以及两个界面之间渗透传输的显着差异,包括 hBN 上的扩散渗透流和电流反转,但在石墨烯上则不然。我们可以使用基于表面离子和水吸附特征长度尺度的简单模型来合理化三种渗透响应的结果,这在石墨烯和 hBN 上有很大不同。我们的工作提供了对二维材料上水性电解质的结构和渗透传输的基本见解,并探索了有效水淡化和渗透能转换的替代途径。










































 京公网安备 11010802027423号
京公网安备 11010802027423号