当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decarboxylative Radical Addition to Methylideneoxazolidinones for Stereocontrolled Synthesis of Selectively Protected Diamino Diacids
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.orglett.1c02684 Yu-Ting Hsiao 1 , Jonathan Beadle 1 , Cameron Pascoe 1 , Ritesh Annadate 1 , John C Vederas 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.orglett.1c02684 Yu-Ting Hsiao 1 , Jonathan Beadle 1 , Cameron Pascoe 1 , Ritesh Annadate 1 , John C Vederas 1
Affiliation
|
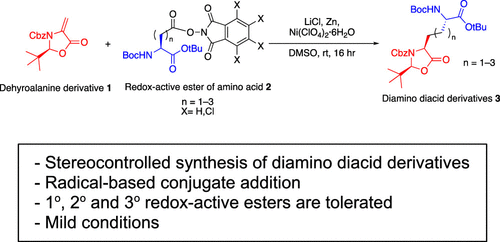
Syntheses of stereochemically pure and selectively protected diamino diacids can be achieved by redox decarboxylation of distal N-hydroxyphthalimide esters of protected aspartic, glutamic or α-aminoadipic acids via radical addition to methylideneoxazolidinones. The products are useful for solid-supported syntheses of robust bioactive carbocyclic peptide analogs. Yields of reactive primary radical addition are superior to those of more stabilized radicals, and the reaction fails if the alkylideneoxazolidinone has a methyl substituent on its terminus (i.e., 13a/13b).
中文翻译:

亚甲基恶唑烷酮的脱羧自由基加成用于立体控制合成选择性保护的二氨基二酸
立体化学纯和选择性保护的二氨基二酸的合成可以通过受保护的天冬氨酸、谷氨酸或 α-氨基己二酸的远端N-羟基邻苯二甲酰亚胺酯通过自由基加成到亚甲基恶唑烷酮的氧化还原脱羧来实现。这些产品可用于强生物活性碳环肽类似物的固体支持合成。反应性伯自由基加成的产率优于更稳定的自由基的产率,如果亚烷基恶唑烷酮在其末端(即13a / 13b)具有甲基取代基,则反应失败。
更新日期:2021-09-17
中文翻译:

亚甲基恶唑烷酮的脱羧自由基加成用于立体控制合成选择性保护的二氨基二酸
立体化学纯和选择性保护的二氨基二酸的合成可以通过受保护的天冬氨酸、谷氨酸或 α-氨基己二酸的远端N-羟基邻苯二甲酰亚胺酯通过自由基加成到亚甲基恶唑烷酮的氧化还原脱羧来实现。这些产品可用于强生物活性碳环肽类似物的固体支持合成。反应性伯自由基加成的产率优于更稳定的自由基的产率,如果亚烷基恶唑烷酮在其末端(即13a / 13b)具有甲基取代基,则反应失败。










































 京公网安备 11010802027423号
京公网安备 11010802027423号