当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational insight into networking H-bonds in open and cyclic forms of glucose
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-09-06 , DOI: 10.1002/poc.4285 Zahrabatoul Mosapour Kotena 1 , Alireza Fattahi 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-09-06 , DOI: 10.1002/poc.4285 Zahrabatoul Mosapour Kotena 1 , Alireza Fattahi 1
Affiliation
|
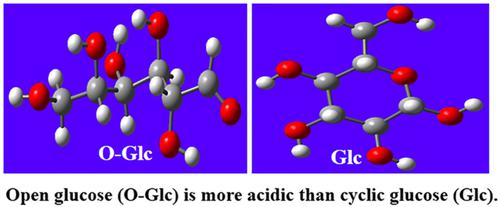
We have studied the intramolecular H-bonds existing in cyclic and open forms of glucose using B3LYP/6-311++G(d,p) level, AIM, and NBO methods. The theoretical results indicated that based on acidity values, (ΔHacid), glucose in the open form is more acidic than cyclic form. The acidity values for open and cyclic glucose (332 and 338 kcal/mol) exhibit significantly lower values (i.e., stronger acid) than the reported acidity values for α-/ß-anomers of D-glucopyranose and simple alcohols. Because their conjugate bases are more stabilized through trifurcated and bifurcated intramolecular H-bonds. AIM analysis showed normal H-bonds in the conjugate bases of open glucose (O-Glc), bifurcated, and normal H-bonds in the conjugate base of cyclic glucose. In the conjugate base of glucose, the O–H···O bonds are categorized as mostly electrostatic and strong, whereas multiple interactions including C–H···O, C–H···H–C (dihydrogen bonding), C–H···C–H, and C=O···H are categorized as weak H-bonds. The NBO results confirm that the O–H···O intramolecular H-bonds should be the strongest among all H-bonds existing within glucose.
中文翻译:

对葡萄糖的开放和循环形式的网络 H 键的计算洞察
我们使用 B3LYP/6-311++G(d,p) 水平、AIM 和 NBO 方法研究了葡萄糖的环状和开放形式中存在的分子内 H 键。理论结果表明,基于酸度值,( ΔH酸),开环形式的葡萄糖比环状形式的葡萄糖酸性更强。开环葡萄糖和环状葡萄糖的酸度值(332 和 338 kcal/mol)显示出比 D-吡喃葡萄糖和简单醇的 α-/β-端基异构体报告的酸度值低得多的值(即,更强的酸)。因为它们的共轭碱基通过三分叉和分叉的分子内 H 键更加稳定。AIM 分析显示在开放葡萄糖 (O-Glc) 的共轭碱基中存在正常的 H 键,在环状葡萄糖的共轭碱基中存在分叉和正常的 H 键。在葡萄糖的共轭基中,O-H···O键主要归类为静电和强键,而多种相互作用包括C-H···O,C-H···H-C(二氢键), C–H…C–H 和 C=O…H 被归类为弱氢键。
更新日期:2021-09-06
中文翻译:

对葡萄糖的开放和循环形式的网络 H 键的计算洞察
我们使用 B3LYP/6-311++G(d,p) 水平、AIM 和 NBO 方法研究了葡萄糖的环状和开放形式中存在的分子内 H 键。理论结果表明,基于酸度值,( ΔH酸),开环形式的葡萄糖比环状形式的葡萄糖酸性更强。开环葡萄糖和环状葡萄糖的酸度值(332 和 338 kcal/mol)显示出比 D-吡喃葡萄糖和简单醇的 α-/β-端基异构体报告的酸度值低得多的值(即,更强的酸)。因为它们的共轭碱基通过三分叉和分叉的分子内 H 键更加稳定。AIM 分析显示在开放葡萄糖 (O-Glc) 的共轭碱基中存在正常的 H 键,在环状葡萄糖的共轭碱基中存在分叉和正常的 H 键。在葡萄糖的共轭基中,O-H···O键主要归类为静电和强键,而多种相互作用包括C-H···O,C-H···H-C(二氢键), C–H…C–H 和 C=O…H 被归类为弱氢键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号