当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Characterization of a Putatively Chitin-Active LPMO Reveals a Preference for Soluble Substrates and Absence of Monooxygenase Activity
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acscatal.1c03344 Lukas Rieder 1 , Dejan Petrović 1 , Priit Väljamäe 2 , Vincent G H Eijsink 1 , Morten Sørlie 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acscatal.1c03344 Lukas Rieder 1 , Dejan Petrović 1 , Priit Väljamäe 2 , Vincent G H Eijsink 1 , Morten Sørlie 1
Affiliation
|
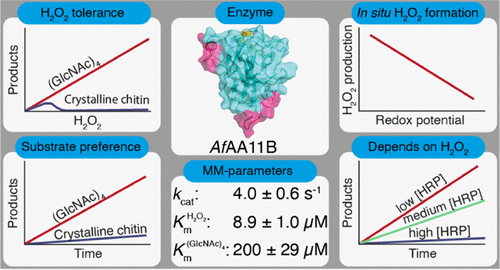
Enzymes known as lytic polysaccharide monooxygenases (LPMOs) are recognized as important contributors to aerobic enzymatic degradation of recalcitrant polysaccharides such as chitin and cellulose. LPMOs are remarkably abundant in nature, with some fungal species possessing more than 50 LPMO genes, and the biological implications of this diversity remain enigmatic. For example, chitin-active LPMOs have been encountered in biological niches where chitin conversion does not seem to take place. We have carried out an in-depth kinetic characterization of a putatively chitin-active LPMO from Aspergillus fumigatus (AfAA11B), which, as we show here, has multiple unusual properties, such as a low redox potential and high oxidase activity. Furthermore, AfAA11B is hardly active on chitin, while being very active on soluble oligomers of N-acetylglucosamine. In the presence of chitotetraose, the enzyme can withstand considerable amounts of H2O2, which it uses to efficiently and stoichiometrically convert this substrate. The unique properties of AfAA11B allowed experiments showing that it is a strict peroxygenase and does not catalyze a monooxygenase reaction. This study shows that nature uses LPMOs for breaking glycosidic bonds in non-polymeric substrates in reactions that depend on H2O2. The quest for the true substrates of these enzymes, possibly carbohydrates in the cell wall of the fungus or its competitors, will be of major interest.
中文翻译:

推定几丁质活性 LPMO 的动力学表征揭示了对可溶性底物的偏好和单加氧酶活性的缺失
被称为裂解多糖单加氧酶 (LPMO) 的酶被认为是顽固多糖(如几丁质和纤维素)有氧酶促降解的重要贡献者。LPMO 在自然界中非常丰富,一些真菌物种拥有 50 多个 LPMO 基因,这种多样性的生物学意义仍然是个谜。例如,在似乎没有发生几丁质转化的生物生态位中遇到了几丁质活性 LPMO。我们对来自烟曲霉( Af AA11B)的假定几丁质活性LPMO进行了深入的动力学表征,正如我们在此展示的,它具有多种不寻常的特性,例如低氧化还原电位和高氧化酶活性。此外,AfAA11B 对几丁质几乎没有活性,而对N-乙酰氨基葡糖的可溶性低聚物非常活跃。在壳四糖的存在下,该酶可以承受大量的 H 2 O 2,它用来有效地和化学计量地转化该底物。Af AA11B的独特性质使实验表明它是一种严格的过氧化酶,不催化单加氧酶反应。这项研究表明,在依赖 H 2 O 2 的反应中,大自然使用 LPMO 来破坏非聚合物底物中的糖苷键. 寻找这些酶的真正底物,可能是真菌或其竞争者细胞壁中的碳水化合物,将引起重大兴趣。
更新日期:2021-09-17
中文翻译:

推定几丁质活性 LPMO 的动力学表征揭示了对可溶性底物的偏好和单加氧酶活性的缺失
被称为裂解多糖单加氧酶 (LPMO) 的酶被认为是顽固多糖(如几丁质和纤维素)有氧酶促降解的重要贡献者。LPMO 在自然界中非常丰富,一些真菌物种拥有 50 多个 LPMO 基因,这种多样性的生物学意义仍然是个谜。例如,在似乎没有发生几丁质转化的生物生态位中遇到了几丁质活性 LPMO。我们对来自烟曲霉( Af AA11B)的假定几丁质活性LPMO进行了深入的动力学表征,正如我们在此展示的,它具有多种不寻常的特性,例如低氧化还原电位和高氧化酶活性。此外,AfAA11B 对几丁质几乎没有活性,而对N-乙酰氨基葡糖的可溶性低聚物非常活跃。在壳四糖的存在下,该酶可以承受大量的 H 2 O 2,它用来有效地和化学计量地转化该底物。Af AA11B的独特性质使实验表明它是一种严格的过氧化酶,不催化单加氧酶反应。这项研究表明,在依赖 H 2 O 2 的反应中,大自然使用 LPMO 来破坏非聚合物底物中的糖苷键. 寻找这些酶的真正底物,可能是真菌或其竞争者细胞壁中的碳水化合物,将引起重大兴趣。









































 京公网安备 11010802027423号
京公网安备 11010802027423号