当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate-Controlled Chemo-/Enantioselective Synthesis of α-Benzylated Enals and Chiral Cyclopropane-Fused 2-Chromanone Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-06 , DOI: 10.1002/adsc.202100613 Huimyoung Byeon 1 , Sunghyeon Ryu 1 , Eun Jeong Yoo 2 , Jung Woon Yang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-06 , DOI: 10.1002/adsc.202100613 Huimyoung Byeon 1 , Sunghyeon Ryu 1 , Eun Jeong Yoo 2 , Jung Woon Yang 1
Affiliation
|
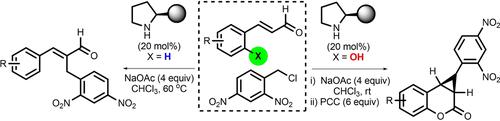
Substrate-controlled cascade reactions between α,β-unsaturated aldehydes or their analogues and 2,4-dinitrobenzyl chloride in the presence of a chiral secondary amine as the catalyst and base were developed, to obtain a broad spectrum of α-benzylated enals and enantioenriched cyclopropane-fused chroman-2-one derivatives. The cyclopropane-tethered iminium ion clearly served as a key intermediate in these reactions to trigger stereochemical outcomes, one of which was supported by a control experiment.
中文翻译:

α-苄基化烯醛和手性环丙烷稠合 2-色甘酮衍生物的底物控制化学/对映选择性合成
在手性仲胺作为催化剂和碱存在下,开发了 α,β-不饱和醛或其类似物与 2,4-二硝基苄基氯之间的底物控制级联反应,以获得广谱的 α-苄基化烯醛和对映体富集环丙烷稠合的chroman-2-one衍生物。环丙烷束缚的亚胺离子显然是这些反应中触发立体化学结果的关键中间体,其中一个得到了对照实验的支持。
更新日期:2021-09-06
中文翻译:

α-苄基化烯醛和手性环丙烷稠合 2-色甘酮衍生物的底物控制化学/对映选择性合成
在手性仲胺作为催化剂和碱存在下,开发了 α,β-不饱和醛或其类似物与 2,4-二硝基苄基氯之间的底物控制级联反应,以获得广谱的 α-苄基化烯醛和对映体富集环丙烷稠合的chroman-2-one衍生物。环丙烷束缚的亚胺离子显然是这些反应中触发立体化学结果的关键中间体,其中一个得到了对照实验的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号