当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Chip Enhanced Time-Gated Luminescent CRISPR-Cas12a Biosensors under Functional DNA Regulation
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.analchem.1c01403 Cheng-Yu Li 1 , Bei Zheng 2, 3 , Li-Li Lu 1, 4 , Wen-Kai Fang 2 , Ming-Qiu Zheng 2 , Jia-Ling Gao 1 , Liu Yuheng 1 , Dai-Wen Pang 5 , Hong-Wu Tang 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.analchem.1c01403 Cheng-Yu Li 1 , Bei Zheng 2, 3 , Li-Li Lu 1, 4 , Wen-Kai Fang 2 , Ming-Qiu Zheng 2 , Jia-Ling Gao 1 , Liu Yuheng 1 , Dai-Wen Pang 5 , Hong-Wu Tang 2
Affiliation
|
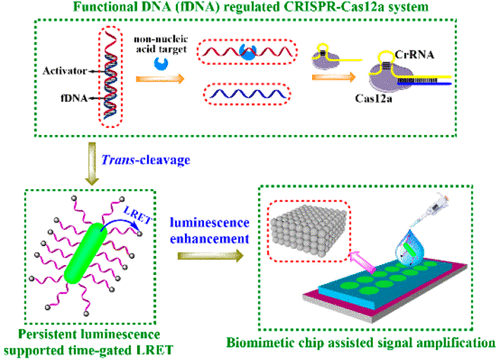
Despite that the currently discovered CRISPR-Cas12a system is beneficial for improving the detection accuracy and design flexibility of luminescent biosensors, there are still challenges to extend target species and strengthen adaptability in complicated biological media. To conquer these obstacles, we present here some useful strategies. For the former, the limitation to nucleic acids assay is broken through by introducing a simple functional DNA regulation pathway to activate the unique trans-cleavage effect of this CRISPR system, under which the expected biosensors are capable of effectively transducing a protein (employing dual aptamers) and a metal ion (employing DNAzyme). For the latter, a time-gated luminescence resonance energy transfer imaging manner using a long-persistent nanophosphor as the energy donor is performed to completely eliminate the background interference and a nature-inspired biomimetic periodic chip constructed by photonic crystals is further combined to enhance the persistent luminescence. In line with the above efforts, the improved CRISPR-Cas12a luminescent biosensor not only exhibits a sound analysis performance toward the model targets (carcinoembryonic antigen and Na+) but also owns a strong anti-interference feature to actualize accurate sensing in human plasma samples, offering a new and applicative analytical tool for laboratory medicine.
中文翻译:

功能性DNA调控下的仿生芯片增强型时间门控发光CRISPR-Cas12a生物传感器
尽管目前发现的 CRISPR-Cas12a 系统有利于提高发光生物传感器的检测精度和设计灵活性,但在扩展目标物种和增强复杂生物介质中的适应性方面仍然存在挑战。为了克服这些障碍,我们在此介绍一些有用的策略。对于前者,通过引入简单的功能性 DNA 调控途径来激活该 CRISPR 系统独特的反式切割效应,突破了对核酸检测的限制,在这种情况下,预期的生物传感器能够有效地转导蛋白质(采用双适体) ) 和金属离子(使用 DNAzyme)。对于后者,采用长余辉纳米磷光体作为能量供体的时间门控发光共振能量转移成像方式,彻底消除背景干扰,并进一步结合光子晶体构建的仿生仿生周期芯片,增强余辉发光。根据上述努力,改进的 CRISPR-Cas12a 发光生物传感器不仅对模型目标(癌胚抗原和 Na+ ) 还具有强大的抗干扰特性,可实现人体血浆样本的精确传感,为实验室医学提供了一种新的、适用的分析工具。
更新日期:2021-09-21
中文翻译:

功能性DNA调控下的仿生芯片增强型时间门控发光CRISPR-Cas12a生物传感器
尽管目前发现的 CRISPR-Cas12a 系统有利于提高发光生物传感器的检测精度和设计灵活性,但在扩展目标物种和增强复杂生物介质中的适应性方面仍然存在挑战。为了克服这些障碍,我们在此介绍一些有用的策略。对于前者,通过引入简单的功能性 DNA 调控途径来激活该 CRISPR 系统独特的反式切割效应,突破了对核酸检测的限制,在这种情况下,预期的生物传感器能够有效地转导蛋白质(采用双适体) ) 和金属离子(使用 DNAzyme)。对于后者,采用长余辉纳米磷光体作为能量供体的时间门控发光共振能量转移成像方式,彻底消除背景干扰,并进一步结合光子晶体构建的仿生仿生周期芯片,增强余辉发光。根据上述努力,改进的 CRISPR-Cas12a 发光生物传感器不仅对模型目标(癌胚抗原和 Na+ ) 还具有强大的抗干扰特性,可实现人体血浆样本的精确传感,为实验室医学提供了一种新的、适用的分析工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号