当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aza-Matteson Reactions via Controlled Mono- and Double-Methylene Insertions into Nitrogen–Boron Bonds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-07 , DOI: 10.1021/jacs.1c06186 Qiqiang Xie 1 , Guangbin Dong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-07 , DOI: 10.1021/jacs.1c06186 Qiqiang Xie 1 , Guangbin Dong 1
Affiliation
|
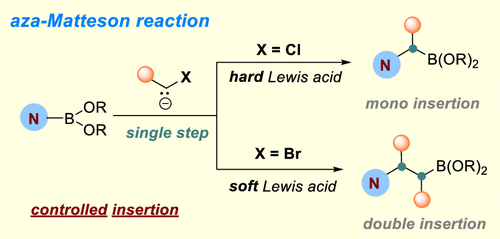
Boron-homologation reactions represent an efficient and programmable approach to prepare alkylboronates, which are valuable and versatile synthetic intermediates. The typical boron-homologation reaction, also known as the Matteson reaction, involves formal carbenoid insertions into C–B bonds. Here we report the development of aza-Matteson reactions via carbenoid insertions into the N–B bonds of aminoboranes. By changing the leaving groups of the carbenoids and altering Lewis acid activators, selective mono- and double-methylene insertions can be realized to access various α- and β-boron-substituted tertiary amines, respectively, from common secondary amines. The derivatization of complex amine-containing bioactive molecules, diverse functionalization of the boronate products, and sequential insertions of different carbenoids have also been achieved.
中文翻译:

通过受控的单亚甲基和双亚甲基插入氮-硼键的 Aza-Matteson 反应
硼同系化反应代表了一种制备烷基硼酸盐的有效且可编程的方法,烷基硼酸盐是一种有价值且用途广泛的合成中间体。典型的硼同系化反应,也称为 Matteson 反应,涉及到 C-B 键的正式卡宾插入。在这里,我们报告了通过将类卡宾插入氨基硼烷的 N-B 键来发展 aza-Matteson 反应。通过改变类胡萝卜素的离去基团和改变路易斯酸活化剂,可以实现选择性的单亚甲基和双亚甲基插入,从而分别从常见的仲胺中获得各种α-和β-硼取代的叔胺。复杂的含胺生物活性分子的衍生化,硼酸盐产品的多样化功能化,
更新日期:2021-09-15
中文翻译:

通过受控的单亚甲基和双亚甲基插入氮-硼键的 Aza-Matteson 反应
硼同系化反应代表了一种制备烷基硼酸盐的有效且可编程的方法,烷基硼酸盐是一种有价值且用途广泛的合成中间体。典型的硼同系化反应,也称为 Matteson 反应,涉及到 C-B 键的正式卡宾插入。在这里,我们报告了通过将类卡宾插入氨基硼烷的 N-B 键来发展 aza-Matteson 反应。通过改变类胡萝卜素的离去基团和改变路易斯酸活化剂,可以实现选择性的单亚甲基和双亚甲基插入,从而分别从常见的仲胺中获得各种α-和β-硼取代的叔胺。复杂的含胺生物活性分子的衍生化,硼酸盐产品的多样化功能化,











































 京公网安备 11010802027423号
京公网安备 11010802027423号