当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Electron Transfer and Substrate Binding Support [NiFe3S4] Ferredoxin as a Protein-Based Model for [NiFe] Carbon Monoxide Dehydrogenase
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.inorgchem.1c01323 Luke C Lewis 1 , Hannah S Shafaat 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.inorgchem.1c01323 Luke C Lewis 1 , Hannah S Shafaat 1
Affiliation
|
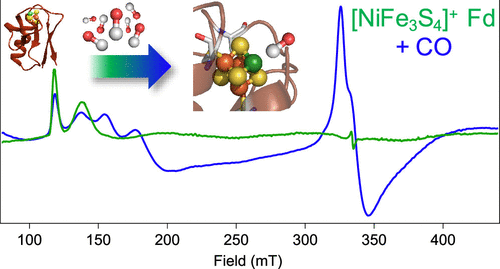
The nickel–iron carbon monoxide dehydrogenase (CODH) enzyme catalyzes the reversible and selective interconversion of carbon dioxide (CO2) to carbon monoxide (CO) with high rates and negligible overpotential. Despite decades of research, many questions remain about this complex metalloenzyme system. A simplified model enzyme could provide substantial insight into biological carbon cycling. Here, we demonstrate reversible electron transfer and binding of both CO and cyanide, a substrate and an inhibitor of CODH, respectively, in a Pyrococcus furiosus (Pf) ferredoxin (Fd) protein that has been reconstituted with a nickel–iron sulfide cluster ([NiFe3S4] Fd). The [NiFe3S4] cluster mimics the core of the native CODH active site and thus serves as a protein-based structural model of the CODH subsite. Notably, despite binding cyanide, no CO binding is observed for the physiological [Fe4S4] clusters in Pf Fd, providing chemical rationale underlying the evolution of a site-differentiated cluster for substrate conversion in native CODH. The demonstration of a substrate-binding metalloprotein model of CODH sets the stage for high-resolution spectroscopic and mechanistic studies correlating the subsite structure and function, ultimately guiding the design of anthropogenic catalysts that harness the advantages of CODH for effective CO2 reduction.
中文翻译:

可逆电子转移和底物结合支持 [NiFe3S4] 铁氧还蛋白作为 [NiFe] 一氧化碳脱氢酶的基于蛋白质的模型
镍铁一氧化碳脱氢酶 (CODH) 酶以高速率和可忽略的过电位催化二氧化碳 (CO 2 ) 向一氧化碳 (CO)的可逆和选择性互变。尽管进行了数十年的研究,但关于这种复杂的金属酶系统仍然存在许多问题。一种简化的模型酶可以提供对生物碳循环的深入了解。这里,我们证明可逆电子转移和CO和氰化物,基板和CODH,分别的抑制剂的结合,在激烈热球菌(Pf的)铁氧还蛋白(FD)蛋白已重构与镍-铁硫化物簇([ NiFe 3 S 4 ] Fd)。[NiFe 3 S 4] 簇模仿天然 CODH 活性位点的核心,因此可作为 CODH 亚位点的基于蛋白质的结构模型。值得注意的是,尽管结合氰化物,在Pf Fd 中没有观察到生理 [Fe 4 S 4 ] 簇的CO 结合,这为天然 CODH 中底物转化的位点分化簇的进化提供了化学原理。CODH 底物结合金属蛋白模型的演示为与亚位点结构和功能相关的高分辨率光谱和机械研究奠定了基础,最终指导了利用 CODH 的优势有效减少CO 2的人为催化剂的设计。
更新日期:2021-09-20
中文翻译:

可逆电子转移和底物结合支持 [NiFe3S4] 铁氧还蛋白作为 [NiFe] 一氧化碳脱氢酶的基于蛋白质的模型
镍铁一氧化碳脱氢酶 (CODH) 酶以高速率和可忽略的过电位催化二氧化碳 (CO 2 ) 向一氧化碳 (CO)的可逆和选择性互变。尽管进行了数十年的研究,但关于这种复杂的金属酶系统仍然存在许多问题。一种简化的模型酶可以提供对生物碳循环的深入了解。这里,我们证明可逆电子转移和CO和氰化物,基板和CODH,分别的抑制剂的结合,在激烈热球菌(Pf的)铁氧还蛋白(FD)蛋白已重构与镍-铁硫化物簇([ NiFe 3 S 4 ] Fd)。[NiFe 3 S 4] 簇模仿天然 CODH 活性位点的核心,因此可作为 CODH 亚位点的基于蛋白质的结构模型。值得注意的是,尽管结合氰化物,在Pf Fd 中没有观察到生理 [Fe 4 S 4 ] 簇的CO 结合,这为天然 CODH 中底物转化的位点分化簇的进化提供了化学原理。CODH 底物结合金属蛋白模型的演示为与亚位点结构和功能相关的高分辨率光谱和机械研究奠定了基础,最终指导了利用 CODH 的优势有效减少CO 2的人为催化剂的设计。









































 京公网安备 11010802027423号
京公网安备 11010802027423号