当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic and computational studies on the binding interaction between gallic acid and Pin1
Luminescence ( IF 3.2 ) Pub Date : 2021-09-07 , DOI: 10.1002/bio.4138 Guo Fei Zhu 1 , Shao Li Lyu 2 , Yang Liu 3 , Chao Ma 1 , Wang Wang 3
Luminescence ( IF 3.2 ) Pub Date : 2021-09-07 , DOI: 10.1002/bio.4138 Guo Fei Zhu 1 , Shao Li Lyu 2 , Yang Liu 3 , Chao Ma 1 , Wang Wang 3
Affiliation
|
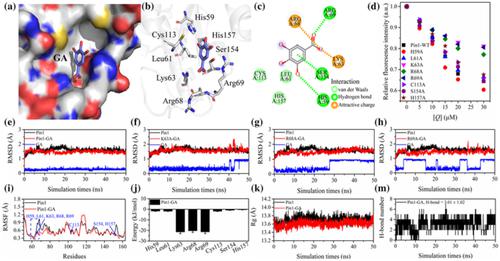
Gallic acid (GA) is a natural ingredient in functional foods, which has various health-promoting and antitumour effects. Peptidyl-prolyl cis/trans isomerase Pin1 plays an important role in preventing the development of some malignant tumours. However, whether there was an interaction between Pin1 and GA remains unknown. In this work, the binding information of GA and Pin1 was investigated systematically using multiple spectral and computational methods. GA bound to Pin1 directly with moderate binding affinity in the order of 104 mol/L, therefore decreasing the activity of Pin1. Also, the binding process of GA to Pin1 was driven through weak van der Waals forces, hydrogen bonds, and electrostatic forces. In addition, the important residues Lys63, Arg68, and Arg69 played a significant role in maintaining the binding stability between Pin1 and GA. Interestingly, GA reduced the activity of Pin1 by affecting its conformational characteristics. Our present work showed that GA binds to Pin1 and inhibits its activity, affecting its structural and functional properties, which may contribute to the therapy of Pin1-related diseases.
中文翻译:

没食子酸与Pin1结合相互作用的光谱和计算研究
没食子酸(GA)是功能性食品中的天然成分,具有多种保健和抗肿瘤作用。肽基-脯氨酰顺/反异构酶Pin1在预防某些恶性肿瘤的发展中起重要作用。然而,Pin1 和 GA 之间是否存在相互作用仍然未知。在这项工作中,使用多种光谱和计算方法系统地研究了 GA 和 Pin1 的结合信息。GA 以 10 4量级的中等结合亲和力直接与 Pin1 结合 mol/L,因此降低了 Pin1 的活性。此外,GA 与 Pin1 的结合过程是通过弱范德华力、氢键和静电力驱动的。此外,重要的残基 Lys63、Arg68 和 Arg69 在维持 Pin1 和 GA 之间的结合稳定性方面发挥了重要作用。有趣的是,GA 通过影响其构象特征降低了 Pin1 的活性。我们目前的工作表明,GA 与 Pin1 结合并抑制其活性,影响其结构和功能特性,这可能有助于 Pin1 相关疾病的治疗。
更新日期:2021-09-07
中文翻译:

没食子酸与Pin1结合相互作用的光谱和计算研究
没食子酸(GA)是功能性食品中的天然成分,具有多种保健和抗肿瘤作用。肽基-脯氨酰顺/反异构酶Pin1在预防某些恶性肿瘤的发展中起重要作用。然而,Pin1 和 GA 之间是否存在相互作用仍然未知。在这项工作中,使用多种光谱和计算方法系统地研究了 GA 和 Pin1 的结合信息。GA 以 10 4量级的中等结合亲和力直接与 Pin1 结合 mol/L,因此降低了 Pin1 的活性。此外,GA 与 Pin1 的结合过程是通过弱范德华力、氢键和静电力驱动的。此外,重要的残基 Lys63、Arg68 和 Arg69 在维持 Pin1 和 GA 之间的结合稳定性方面发挥了重要作用。有趣的是,GA 通过影响其构象特征降低了 Pin1 的活性。我们目前的工作表明,GA 与 Pin1 结合并抑制其活性,影响其结构和功能特性,这可能有助于 Pin1 相关疾病的治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号