International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2021-09-06 , DOI: 10.1016/j.ijpharm.2021.121079 Khushali Parekh 1 , Kartik Hariharan 1 , Zhi Qu 2 , Prarthana Rewatkar 2 , Yuxue Cao 2 , Md Moniruzzaman 3 , Preeti Pandey 2 , Amirali Popat 3 , Tejal Mehta 1
|
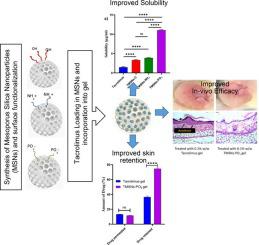
Atopic dermatitis (AD) is a repetitive inflammatory skin disorder with limited treatment options. Innovative targeted therapies are gaining significant interest and momentum towards disease control including better ways to deliver drugs topically. Tacrolimus is one such compound which is used to manage moderate to severe AD without causing atrophy which is one of the common side effects of steroids. However, Tacrolimus suffers from poor solubility and retention in the skin when used alone in hydrogel. Therefore, we have prepared Tacrolimus loaded mesoporous silica nanoparticles (TMSNs) to overcome the issues related to its solubility and effective topical delivery. Mesoporous silica nanoparticles (MSNs) were synthesized using sol gel technique and surface functionalized using amino (–NH2+) and phosphonate (-PO3-) groups. Tacrolimus was loaded into MSNs and the particles were characterized for particle size (TEM and DLS), zeta potential (DLS), solubility studies, FTIR, TGA, XRD, BET and cytotoxicity studies. Water solubility of Tacrolimus was increased by 7 folds with phosphonate functionalized MSNs compared to free Tacrolimus. Further the TMSNs were incorporated in to carbopol gel, and the gel formulation was evaluated for various gel characterization tests (pH, spreadability, viscosity), in vitro tests (drug release, permeability studies) and in vivo tests (skin irritation study and efficacy studies) using 1-Fluoro-2,4-dinitrobenzene (DNFB) induced dermatitis in Balb/c mice. Results of in vitro and in vivo study showed that TMSNs loaded gel showed significantly higher amount of Tacrolimus retained (ex vivo – rat skin) and much higher reduction in ear thickness and improved histology (in vivo - in mice). Our data collectively suggest that MSNs incorporated hydrogel as a promising new formulation strategy for topical delivery of poorly soluble drugs.
中文翻译:

他克莫司包封的介孔二氧化硅纳米粒子嵌入水凝胶治疗特应性皮炎
特应性皮炎 (AD) 是一种治疗选择有限的重复性炎症性皮肤病。创新的靶向疗法在疾病控制方面获得了极大的兴趣和动力,包括更好的局部给药方法。他克莫司就是一种这样的化合物,用于治疗中度至重度 AD 而不会引起萎缩,这是类固醇的常见副作用之一。然而,当他克莫司单独用于水凝胶时,他克莫司的溶解性和在皮肤中的滞留性较差。因此,我们制备了载有他克莫司的介孔二氧化硅纳米粒子 (TMSN),以克服与其溶解性和有效局部给药相关的问题。介孔二氧化硅纳米粒子 (MSN) 使用溶胶凝胶技术合成,并使用氨基 (–NH 2 +) 和膦酸酯 (-PO 3 - ) 基团。他克莫司加载到 MSN 中,并对颗粒进行粒度(TEM 和 DLS)、zeta 电位 (DLS)、溶解度研究、FTIR、TGA、XRD、BET 和细胞毒性研究的表征。与游离的他克莫司相比,使用膦酸盐功能化的 MSN,他克莫司的水溶性增加了 7 倍。此外,将 TMSN 加入卡波姆凝胶中,并针对各种凝胶特性测试(pH、铺展性、粘度)、体外测试(药物释放、渗透性研究)和体内测试(皮肤刺激研究和功效研究)对凝胶配方进行评估) 在 Balb/c 小鼠中使用 1-氟-2,4-二硝基苯 (DNFB) 诱发的皮炎。结果在体外和体内研究表明,加载 TMSNs 的凝胶显示出显着更高的他克莫司保留量(离体- 大鼠皮肤),耳朵厚度减少更多,组织学改善(体内- 小鼠)。我们的数据共同表明,MSNs 将水凝胶作为一种有前途的新配方策略,用于局部递送难溶性药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号