Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-09-06 , DOI: 10.1016/j.jece.2021.106305 Aftab Aslam Parwaz Khan 1 , Mohammed Nazim 2 , Abdullah M. Asiri 1, 3
|
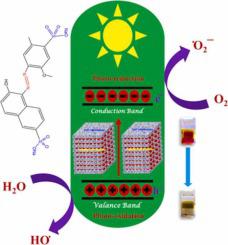
In this work, an eco-friendly, facile, and efficient method has been applied for CuO nanostructures (NSs) synthesis using ascorbic acid as capping agent. For surface morphology, FESEM analysis was applied to reveal nanostructure with ~100 nm length, and ~90 nm width morphology which was also supported by TEM images of CuO. Thus obtained CuO NSs resulted in an optical energy having ~1.36 eV band gap obtained from Tauc plots of UV–visible plots because of strong quantum confinement effect of nanoparticles. XRD reveals a monoclinic phase of CuO NSs for which follow the reference JCPDS No. 98–004–3179 (Space group of C 12/c 1) crystal structure with high crystallinity of ~95.94% and crystallite size of ~38 nm. In addition, Williamson-Hall analysis was applied to calculate crystal lattice strain of ~3.01 × 10-3 for CuO catalyst followed by 28.36 m2/g high BET surface area (0.9999 correlation coefficient) having ~14.38 nm average BJH pore size. Furthermore, CuO NSs exhibited ultrafast and high catalytic degradation efficiency of ~90.54% for Allura Red AC dye (AR dye) in 480 s under UV light. The photodegradation kinetics followed a pseudo first-order reaction with rate constant (k) of ~0.0046 s−1 having approximately ~150.65 s of half-life (t1/2) for AR dyes in the aquatic medium as per the Langmuir–Hinshelwood model. As synthesized catalyst, CuO NSs explored good leaf-like morphology with high recycling ability after five degradation cycles of AR dye in aqueous medium.
中文翻译:

氧化铜纳米材料在水介质中高效催化降解有机污染物
在这项工作中,以抗坏血酸作为封端剂,采用了一种环保、简便、高效的方法合成 CuO 纳米结构 (NSs)。对于表面形态,FESEM 分析用于揭示具有~100 nm 长度和~90 nm 宽度形态的纳米结构,这也得到了 CuO 的 TEM 图像的支持。由于纳米粒子的强量子限制效应,因此获得的 CuO NSs 导致具有 ~1.36 eV 带隙的光能,从紫外-可见光图的 Tauc 图中获得。XRD 揭示了 CuO NSs 的单斜相,其遵循参考 JCPDS No. 98-004-3179(C 12/c 1 空间群)晶体结构,具有~95.94% 的高结晶度和~38 nm 的微晶尺寸。此外,应用威廉姆森-霍尔分析来计算~3.01 × 10 -3 的晶格应变对于 CuO 催化剂,其后是 28.36 m 2 /g 的高 BET 表面积(0.9999 相关系数),平均 BJH 孔径约为 14.38 nm。此外,在紫外光下,CuO NSs 在 480 秒内对诱惑红 AC 染料(AR 染料)表现出超快和高催化降解效率约 90.54%。根据Langmuir-Hinshelwood ,光降解动力学遵循具有~0.0046 s -1速率常数(k)的伪一级反应,具有约~150.65 s的水介质中 AR 染料的半衰期(t 1/2)模型。作为合成催化剂,CuO NSs 在 AR 染料在水性介质中经过 5 次降解循环后,探索出良好的叶状形态和高回收能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号