当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Role of H-Bonding in Organic Electrochemistry – From Supramolecular Applications to Mechanistic Investigations
The Chemical Record ( IF 7.0 ) Pub Date : 2021-09-06 , DOI: 10.1002/tcr.202100186 Diane K Smith 1
The Chemical Record ( IF 7.0 ) Pub Date : 2021-09-06 , DOI: 10.1002/tcr.202100186 Diane K Smith 1
Affiliation
|
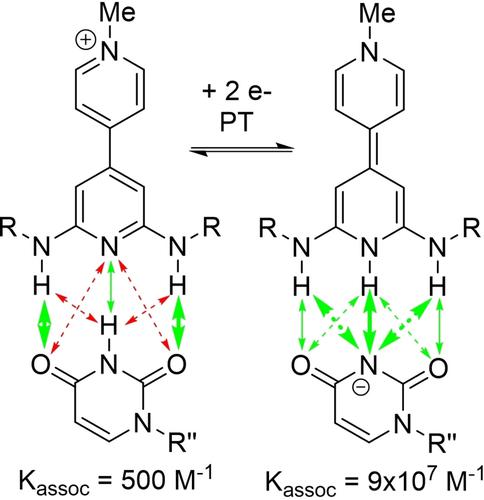
H-bonds can exert a substantial impact on the course of organic electrode reactions due to their ability to stabilize charged intermediates and products formed during these reactions, as well as facilitate proton-coupled electron transfer (PCET) reactions. This has fundamental implications for the mechanism of organic electrode reactions, but also practical impact in supramolecular chemistry and potentially synthetic electrochemistry. My group's main focus has been on the supramolecular applications, using electron transfer to alter the strength of H-bonds to create highly redox-responsive H-bond dimers. Initially we sought to avoid proton transfer because we feared that would lead to irreversible electrochemistry. However, inevitably proton transfer did show up, but, to our surprise, did not lead to irreversible electrochemistry. To explain this, we developed a new mechanism, the “wedge scheme”, that shows how H-bonding can facilitate reversible electron and proton transfer. This insight recently led us to a new PCET-based design strategy for the creation of our most highly redox-responsive H-bond dimers yet.
中文翻译:

探索氢键在有机电化学中的作用——从超分子应用到机理研究
氢键可以对有机电极反应过程产生重大影响,因为它们能够稳定这些反应过程中形成的带电中间体和产物,并促进质子耦合电子转移 (PCET) 反应。这对有机电极反应的机制具有根本意义,但对超分子化学和潜在的合成电化学也有实际影响。我的团队主要关注超分子应用,使用电子转移来改变氢键的强度,以创建高度氧化还原响应的氢键二聚体。最初我们试图避免质子转移,因为我们担心这会导致不可逆的电化学。然而,不可避免的质子转移确实出现了,但令我们惊讶的是,并没有导致不可逆的电化学。为了解释这一点,我们开发了一种新机制,即“楔形方案”,它展示了氢键如何促进可逆电子和质子转移。这一见解最近使我们采用了一种新的基于 PCET 的设计策略,以创建我们迄今为止对氧化还原反应最高的 H 键二聚体。
更新日期:2021-09-19
中文翻译:

探索氢键在有机电化学中的作用——从超分子应用到机理研究
氢键可以对有机电极反应过程产生重大影响,因为它们能够稳定这些反应过程中形成的带电中间体和产物,并促进质子耦合电子转移 (PCET) 反应。这对有机电极反应的机制具有根本意义,但对超分子化学和潜在的合成电化学也有实际影响。我的团队主要关注超分子应用,使用电子转移来改变氢键的强度,以创建高度氧化还原响应的氢键二聚体。最初我们试图避免质子转移,因为我们担心这会导致不可逆的电化学。然而,不可避免的质子转移确实出现了,但令我们惊讶的是,并没有导致不可逆的电化学。为了解释这一点,我们开发了一种新机制,即“楔形方案”,它展示了氢键如何促进可逆电子和质子转移。这一见解最近使我们采用了一种新的基于 PCET 的设计策略,以创建我们迄今为止对氧化还原反应最高的 H 键二聚体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号