当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new 1,4- and 1,5-disubstituted N-ethyl acetate and N-α-butyro-γ-lactone alkylimidazole derivatives as N-acylhomoserine lactone analogs
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-09-06 , DOI: 10.1002/jhet.4356 Zhang Qiang 1 , Si‐Zhe Li 1 , Yves Queneau 1 , Laurent Soulère
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-09-06 , DOI: 10.1002/jhet.4356 Zhang Qiang 1 , Si‐Zhe Li 1 , Yves Queneau 1 , Laurent Soulère
Affiliation
|
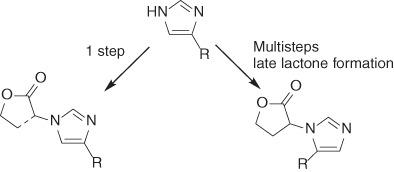
New alkylimidazoles functionalized with a homoserine lactone or an alkyloxycarbonyl moiety have been synthesized as N-acylhomoserine lactones (AHLs) analogs. The 1,4-disubstituted imidazole derivatives were prepared by alkylation of 4(5)-alkylimidazoles with α-bromo-γ-butyrolactone or ethyl α-bromoacetate. An alternative route was preferred for the synthesis of their 1,5-disubstituted counterparts based on the use of a N1-protected alkylimidazole, its alkylation to an N3-imidazolyl-α-acetate and deprotection to the desired 1,5-disubstituted esters and subsequent alkylation of the acetate moiety with cyclic ethylene sulfate followed by acid-catalyzed cyclization. The ability to modulate bacterial quorum sensing of all new compounds was compared to that of previously reported AHL analogs in which the amide bond is replaced by a heterocyclic group.
中文翻译:

合成新的 1,4-和 1,5-二取代 N-乙酸乙酯和 N-α-丁酰-γ-内酯烷基咪唑衍生物作为 N-酰基高丝氨酸内酯类似物
用高丝氨酸内酯或烷氧基羰基部分功能化的新型烷基咪唑已合成为N-酰基高丝氨酸内酯(AHL) 类似物。1,4-二取代咪唑衍生物是通过 4(5)-烷基咪唑与 α-溴-γ-丁内酯或 α-溴乙酸乙酯的烷基化制备的。基于使用N 1 -保护的烷基咪唑,将其烷基化为N 3-咪唑基-α-乙酸酯并脱保护为所需的 1,5-二取代酯,随后用环状硫酸乙烯酯将乙酸酯部分烷基化,然后进行酸催化环化。将调节所有新化合物的细菌群体感应的能力与先前报道的 AHL 类似物进行比较,其中酰胺键被杂环基团取代。
更新日期:2021-09-06
中文翻译:

合成新的 1,4-和 1,5-二取代 N-乙酸乙酯和 N-α-丁酰-γ-内酯烷基咪唑衍生物作为 N-酰基高丝氨酸内酯类似物
用高丝氨酸内酯或烷氧基羰基部分功能化的新型烷基咪唑已合成为N-酰基高丝氨酸内酯(AHL) 类似物。1,4-二取代咪唑衍生物是通过 4(5)-烷基咪唑与 α-溴-γ-丁内酯或 α-溴乙酸乙酯的烷基化制备的。基于使用N 1 -保护的烷基咪唑,将其烷基化为N 3-咪唑基-α-乙酸酯并脱保护为所需的 1,5-二取代酯,随后用环状硫酸乙烯酯将乙酸酯部分烷基化,然后进行酸催化环化。将调节所有新化合物的细菌群体感应的能力与先前报道的 AHL 类似物进行比较,其中酰胺键被杂环基团取代。











































 京公网安备 11010802027423号
京公网安备 11010802027423号