当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning the Solubility of the Herbicide Bentazon: from Salt to Neutral and to Inclusion Complexes
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-09-06 , DOI: 10.1021/acssuschemeng.1c02749 Alessandra Azzali 1 , Simone d’Agostino 1 , Fabrizia Grepioni 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-09-06 , DOI: 10.1021/acssuschemeng.1c02749 Alessandra Azzali 1 , Simone d’Agostino 1 , Fabrizia Grepioni 1
Affiliation
|
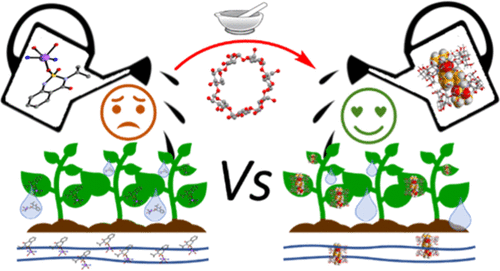
We report on the mechanochemical synthesis of inclusion complexes obtained by reacting the neutral crystalline herbicide bentazon (HBtz) with native cyclodextrins (CDs). The reaction of HBtz with γ-CD resulted in the formation of the inclusion complexes [β-CD·HBtz]·6H2O and [γ-CD·HBtz]·8H2O, which were characterized via a combination of X-ray diffraction, Fourier transform infrared (FTIR) spectroscopy, and calorimetric measurements. No complexation, on the contrary, was achieved upon the reaction of HBtz with α-CD. The salt NaBtz·1.75H2O, widely used as a water-soluble salt of bentazon for the manufacturing of agrochemicals, was also synthesized and structurally characterized, and its solubility and dissolution properties were compared to those of neutral HBtz and of the β-CD and γ-CD inclusion complexes. It was found that the behavior of [β-CD·HBtz]·6H2O and [γ-CD·HBtz]·8H2O in water is similar, with dissolution rates of 28.4 ng·L–1·min–1 and 39.8 ng·L–1·min–1, respectively, and is intermediate between those of NaBtz·1.75H2O (228 ng·L–1·min–1) and neutral bentazon (3.5 ng·L–1·min–1). These results were also compared with those of the dehydrated inclusion complexes, which displayed intermediate dissolution rates between hydrous complexes and NaBtz·1.75H2O. All findings indicate that the inclusion of HBtz in β-CD and γ-CD might represent a viable alternative for the preparation of environmentally friendly agrochemicals with controlled bentazon release to be used in the formulation of herbicides.
中文翻译:

调整除草剂苯达松的溶解度:从盐到中性再到包合物
我们报告了通过中性结晶除草剂苯达松 (HBtz) 与天然环糊精 (CD) 反应获得的包合物的机械化学合成。HBtz与γ-CD反应形成包合物[β-CD·HBtz]·6H 2 O和[γ-CD·HBtz]·8H 2 O,通过X射线结合表征衍射、傅里叶变换红外 (FTIR) 光谱和量热测量。相反,在 HBtz 与 α-CD 反应时没有实现络合。盐NaBtz·1.75H 2O,广泛用作农药制造的苯达松的水溶性盐,也被合成和结构表征,并将其溶解度和溶解特性与中性 HBtz 和 β-CD 和 γ-CD 包合物的溶解度和溶解特性进行比较. 发现[β-CD·HBtz]·6H 2 O和[γ-CD·HBtz]·8H 2 O在水中的行为相似,溶解速率为28.4 ng·L –1 ·min –1和分别为 39.8 ng·L –1 ·min –1,介于 NaBtz·1.75H 2 O (228 ng·L –1 ·min –1 ) 和中性苯达松 (3.5 ng·L –1 ·min –) 之间1)。这些结果还与脱水包合物的结果进行了比较,后者显示出介于含水复合物和 NaBtz·1.75H 2 O之间的中等溶解速率。所有研究结果表明,将 HBtz 包含在 β-CD 和 γ-CD 中可能代表一种可行的替代方法用于制备具有受控苯达松释放的环保农用化学品,用于配制除草剂。
更新日期:2021-09-20
中文翻译:

调整除草剂苯达松的溶解度:从盐到中性再到包合物
我们报告了通过中性结晶除草剂苯达松 (HBtz) 与天然环糊精 (CD) 反应获得的包合物的机械化学合成。HBtz与γ-CD反应形成包合物[β-CD·HBtz]·6H 2 O和[γ-CD·HBtz]·8H 2 O,通过X射线结合表征衍射、傅里叶变换红外 (FTIR) 光谱和量热测量。相反,在 HBtz 与 α-CD 反应时没有实现络合。盐NaBtz·1.75H 2O,广泛用作农药制造的苯达松的水溶性盐,也被合成和结构表征,并将其溶解度和溶解特性与中性 HBtz 和 β-CD 和 γ-CD 包合物的溶解度和溶解特性进行比较. 发现[β-CD·HBtz]·6H 2 O和[γ-CD·HBtz]·8H 2 O在水中的行为相似,溶解速率为28.4 ng·L –1 ·min –1和分别为 39.8 ng·L –1 ·min –1,介于 NaBtz·1.75H 2 O (228 ng·L –1 ·min –1 ) 和中性苯达松 (3.5 ng·L –1 ·min –) 之间1)。这些结果还与脱水包合物的结果进行了比较,后者显示出介于含水复合物和 NaBtz·1.75H 2 O之间的中等溶解速率。所有研究结果表明,将 HBtz 包含在 β-CD 和 γ-CD 中可能代表一种可行的替代方法用于制备具有受控苯达松释放的环保农用化学品,用于配制除草剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号