当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The influence of co-doping on the luminescence and thermoluminescence properties of Cu-containing fluoride borate crystals
CrystEngComm ( IF 2.6 ) Pub Date : 2021-09-06 , DOI: 10.1039/d1ce00556a T. B. Bekker 1, 2, 3 , A. P. Yelisseyev 1, 2 , V. P. Solntsev 1 , A. V. Davydov 1 , T. M. Inerbaev 1, 4 , S. V. Rashchenko 1, 2 , A. I. Kostyukov 2, 5
CrystEngComm ( IF 2.6 ) Pub Date : 2021-09-06 , DOI: 10.1039/d1ce00556a T. B. Bekker 1, 2, 3 , A. P. Yelisseyev 1, 2 , V. P. Solntsev 1 , A. V. Davydov 1 , T. M. Inerbaev 1, 4 , S. V. Rashchenko 1, 2 , A. I. Kostyukov 2, 5
Affiliation
|
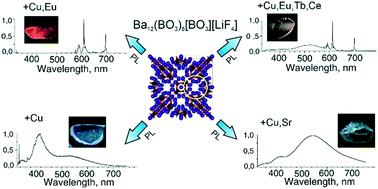
The results of this study provide data on the luminescence and thermoluminescence properties of fluoride borate Ba12(BO3)6[BO3][LiF4] crystals co-doped with copper, rare earth elements, and strontium. The crystals were grown from high temperature solutions. This study revealed a considerable difference in decay time: about 60 ns for Cu+ photoluminescence at 412 nm in Ba12(BO3)6[BO3][LiF4]:Cu crystals after excitation at 263 nm, and about 1.8 ms for Eu3+ photoluminescence at 612 nm in Ba12(BO3)6[BO3][LiF4]:Cu,Eu crystals after excitation at 300, 325, and 395 nm. The absence of short-wavelength luminescence of Cu+ centers in photoluminescence spectra under 325 nm excitation in Ba12(BO3)6[BO3][LiF4]:Cu,Eu and Ba12(BO3)6[BO3][LiF4]:Cu,Eu,Tb,Ce crystals is associated with the nonradiative energy transfer from the excited electronic state of Cu+ ions to the energy levels of the rare earth elements. The thermoluminescence curves of these crystals lack a peak associated with capture centers. Co-doping with copper and strontium (Ba12(BO3)6[BO3][LiF4]:Cu,Sr, P42/mbc, a = 13.5174 (3) Å, c = 14.9399 (3) Å) has a noticeable effect on both photo- and thermoluminescence properties. This fosters the formation of deeper capture centers and promotes an increase in the temperature interval between thermoluminescence peaks, which is essential for the stable storage of dosimetric information. First-principles density functional theory investigation indicates that the presence of monovalent copper ions narrows the bandgap width compared to that of undoped Ba12(BO3)6[BO3][LiF4] crystals due to the transitions from the Cu-3d to Ba-5d levels. The presence of bivalent copper in the structure results in unfilled defect levels inside the bandgap. This contributes to the absorption in the visible range due to the electronic transitions from the O-2p to Cu-3d levels.
中文翻译:

共掺杂对含Cu氟硼酸盐晶体发光和热释光性能的影响
该研究的结果提供了关于与铜、稀土元素和锶共掺杂的氟硼酸盐 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ] 晶体的发光和热释光特性的数据。晶体是从高温溶液中生长出来的。该研究揭示了衰减时间的显着差异:在 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu 晶体中,412 nm 处的Cu +光致发光在 263 nm 处激发后大约为 60 ns ,而对于在 Ba 12 (BO) 中612 nm 处的Eu 3+光致发光3 ) 6 [BO 3 ][LiF 4 ]:在 300、325 和 395 nm 激发后的 Cu、Eu 晶体。在 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu、Eu 和 Ba 12 (BO 3 ) 6 [BO 3 ] 中,在 325 nm 激发下的光致发光光谱中没有 Cu +中心的短波长发光[LiF 4 ]:Cu,Eu,Tb,Ce 晶体与 Cu +激发电子态的非辐射能量转移有关离子达到稀土元素的能级。这些晶体的热释光曲线缺少与捕获中心相关的峰。与铜和锶共掺杂 (Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu,Sr, P 4 2 / mbc , a = 13.5174 (3) Å, c= 14.9399 (3) Å) 对光致发光和热致发光特性都有显着影响。这促进了更深捕获中心的形成,并促进了热释光峰之间温度间隔的增加,这对于剂量学信息的稳定存储至关重要。第一性原理密度泛函理论研究表明,与未掺杂的 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4相比,一价铜离子的存在使带隙宽度变窄] 晶体由于从 Cu-3d 到 Ba-5d 能级的转变。结构中二价铜的存在导致带隙内未填充的缺陷水平。由于从 O-2p 到 Cu-3d 能级的电子跃迁,这有助于可见光范围内的吸收。
更新日期:2021-09-06
中文翻译:

共掺杂对含Cu氟硼酸盐晶体发光和热释光性能的影响
该研究的结果提供了关于与铜、稀土元素和锶共掺杂的氟硼酸盐 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ] 晶体的发光和热释光特性的数据。晶体是从高温溶液中生长出来的。该研究揭示了衰减时间的显着差异:在 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu 晶体中,412 nm 处的Cu +光致发光在 263 nm 处激发后大约为 60 ns ,而对于在 Ba 12 (BO) 中612 nm 处的Eu 3+光致发光3 ) 6 [BO 3 ][LiF 4 ]:在 300、325 和 395 nm 激发后的 Cu、Eu 晶体。在 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu、Eu 和 Ba 12 (BO 3 ) 6 [BO 3 ] 中,在 325 nm 激发下的光致发光光谱中没有 Cu +中心的短波长发光[LiF 4 ]:Cu,Eu,Tb,Ce 晶体与 Cu +激发电子态的非辐射能量转移有关离子达到稀土元素的能级。这些晶体的热释光曲线缺少与捕获中心相关的峰。与铜和锶共掺杂 (Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4 ]:Cu,Sr, P 4 2 / mbc , a = 13.5174 (3) Å, c= 14.9399 (3) Å) 对光致发光和热致发光特性都有显着影响。这促进了更深捕获中心的形成,并促进了热释光峰之间温度间隔的增加,这对于剂量学信息的稳定存储至关重要。第一性原理密度泛函理论研究表明,与未掺杂的 Ba 12 (BO 3 ) 6 [BO 3 ][LiF 4相比,一价铜离子的存在使带隙宽度变窄] 晶体由于从 Cu-3d 到 Ba-5d 能级的转变。结构中二价铜的存在导致带隙内未填充的缺陷水平。由于从 O-2p 到 Cu-3d 能级的电子跃迁,这有助于可见光范围内的吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号