当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison Study of the SCR Performance over Mn–TiO2 and Ce–TiO2 Catalysts: An Experimental and DFT Study
Energy & Fuels ( IF 5.2 ) Pub Date : 2021-09-06 , DOI: 10.1021/acs.energyfuels.1c02170 Wenjie Zhang 1 , Yi Zhong 2 , Qi Wang 3 , Dekui Shen 1 , Guofu Liu 4
Energy & Fuels ( IF 5.2 ) Pub Date : 2021-09-06 , DOI: 10.1021/acs.energyfuels.1c02170 Wenjie Zhang 1 , Yi Zhong 2 , Qi Wang 3 , Dekui Shen 1 , Guofu Liu 4
Affiliation
|
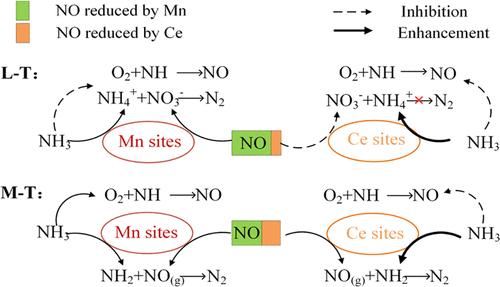
MnOx- and CeOx-based catalysts are the most popular low-temperature selective catalytic reduction (SCR) catalysts for the substitution of the VO5–WO3/TiO2 catalyst. The comparison study was conducted to reveal the inner mechanism for the difference of the SCR performance over Mn–TiO2 and Ce–TiO2 catalysts via the experimental analysis and density-functional theory (DFT) calculations. The results suggested that the inhibited low-temperature NO conversion rate over the Ce–TiO2 catalyst is attributed to the highly inhibited Langmuir–Hinshelwood mechanism (L–H mechanism pathway) due to the insufficient redox properties, restricting the activation of adsorbed NO and the formation of the intermediate NH4NO2 species. As for the middle and high temperatures, the NO conversion rate is the synergistic effect of the inhibited SCR reaction following Eley–Rideal mechanism (E–R pathway) and the C–O reaction rate (catalytic oxidation reaction). The strong interaction between NH3 and the Ce–TiO2 catalyst is the main reason for the inhibition of the kE–R value over the Ce–TiO2 catalyst, inhibiting the reactivity of the adsorbed NH2 with gaseous NO. Meanwhile, the C–O reaction over the Ce–TiO2 catalyst was inhibited by the weakened oxidation properties and lower concentrations of Ce3+/Ce. Therefore, the Ce–TiO2 catalyst presented a slightly lower middle-temperature NO conversion rate compared to the Mn–TiO2 catalyst.
中文翻译:

Mn-TiO2 和 Ce-TiO2 催化剂的 SCR 性能比较研究:实验和 DFT 研究
MnO x - 和CeO x基催化剂是最流行的低温选择性催化还原(SCR)催化剂,用于替代VO 5 –WO 3 /TiO 2催化剂。通过实验分析和密度泛函理论(DFT)计算,对比研究揭示了 Mn-TiO 2和 Ce-TiO 2催化剂SCR 性能差异的内在机制。结果表明,Ce-TiO 2抑制了低温 NO 转化率催化剂归因于高度抑制的 Langmuir-Hinshelwood 机制(L-H 机制途径),因为氧化还原性能不足,限制了吸附的 NO 的活化和中间 NH 4 NO 2物种的形成。对于中高温,NO 转化率是遵循 Eley-Rideal 机制(E-R 途径)和 C-O 反应速率(催化氧化反应)的抑制 SCR 反应的协同效应。NH之间的强相互作用3和Ce的的TiO 2催化剂是用于抑制的主要原因ķ ë-R在铈的TiO值2催化剂,抑制吸附的反应性NH 2与气态 NO。同时,Ce-TiO 2催化剂上的C-O 反应被减弱的氧化性能和较低的Ce 3+ /Ce浓度抑制。因此,与Mn-TiO 2催化剂相比,Ce-TiO 2催化剂的中温NO 转化率略低。
更新日期:2021-09-16
中文翻译:

Mn-TiO2 和 Ce-TiO2 催化剂的 SCR 性能比较研究:实验和 DFT 研究
MnO x - 和CeO x基催化剂是最流行的低温选择性催化还原(SCR)催化剂,用于替代VO 5 –WO 3 /TiO 2催化剂。通过实验分析和密度泛函理论(DFT)计算,对比研究揭示了 Mn-TiO 2和 Ce-TiO 2催化剂SCR 性能差异的内在机制。结果表明,Ce-TiO 2抑制了低温 NO 转化率催化剂归因于高度抑制的 Langmuir-Hinshelwood 机制(L-H 机制途径),因为氧化还原性能不足,限制了吸附的 NO 的活化和中间 NH 4 NO 2物种的形成。对于中高温,NO 转化率是遵循 Eley-Rideal 机制(E-R 途径)和 C-O 反应速率(催化氧化反应)的抑制 SCR 反应的协同效应。NH之间的强相互作用3和Ce的的TiO 2催化剂是用于抑制的主要原因ķ ë-R在铈的TiO值2催化剂,抑制吸附的反应性NH 2与气态 NO。同时,Ce-TiO 2催化剂上的C-O 反应被减弱的氧化性能和较低的Ce 3+ /Ce浓度抑制。因此,与Mn-TiO 2催化剂相比,Ce-TiO 2催化剂的中温NO 转化率略低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号