Optical Materials ( IF 3.9 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.optmat.2021.111541 Jianhe Tang 1 , Yu Liu 2 , Yitong Lin 1 , Xueke Liu 2 , Liang Chen 2 , Congcong Piao 2 , Dawei Fang 2, 3 , Jun Wang 1, 2
|
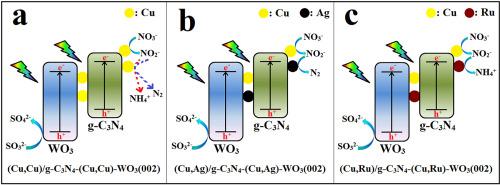
Photocatalysis technology is an effective way for converting nitrate (NO3−) and sulfite (SO32−) in wastewater to pollution-free nitrogen (N2) and exploitable ammonium sulfate ((NH4)2SO4). In this study, three novel photocatalysts, Z-scheme (Cu,Cu)/g–C3N4–(Cu,Cu)-WO3(002), Z-scheme (Cu,Ag)/g–C3N4–(Cu,Ag)-WO3(002) and Z-scheme (Cu,Ru)/g–C3N4–(Cu,Ru)-WO3(002), were prepared by photo-assisted electrostatic self-assembly method. The prepared photocatalysts are synthetically characterized by XRD, SEM, TEM, EDX, XPS, DRS, PL, TPR and EIS. The effects of different co-catalyst combinations on the activity and selectivity of the photocatalysts are evaluated. Also, the impacts of simulated solar light irradiation time, NO3− initial concentration and cycle times on the conversions of NO3− and SO32− are studied. The results display that the Z-scheme (Cu,Ag)/g–C3N4–(Cu,Ag)-WO3(002) photocatalyst can selectively convert NO3− to N2, while the Z-scheme (Cu,Ru)/g–C3N4–(Cu,Ru)-WO3(002) photocatalyst can selectively convert NO3− to NH4+. Nevertheless, the Z-scheme (Cu,Cu)/g–C3N4–(Cu,Cu)-WO3(002) photocatalyst has hardly selectivity for conversion of NO3−. These three photocatalysts all exhibit relatively strong capabilities for converting SO32− to sulfate (SO42−) due to the existence of the highly active (002) plane of WO3. Finally, the possible mechanisms on the photocatalytic conversions of NO3− and SO32− caused by Z-scheme (Cu,M)/g–C3N4–(Cu,M)-WO3(002) (M = Cu, Ag and Ru) photocatalysts are proposed.
中文翻译:

设计和构建多种双助催化剂装饰的 Z 型 g-C3N4/WO3(002) 光催化剂,用于转化硝酸盐和亚硫酸盐
光催化技术是将废水中的硝酸盐(NO 3 -)和亚硫酸盐(SO 3 2-)转化为无污染的氮(N 2)和可利用的硫酸铵((NH 4 ) 2 SO 4)的有效途径。在本研究中,三种新型光催化剂,Z 型 (Cu,Cu)/g–C 3 N 4 –(Cu,Cu)-WO 3 (002)、Z 型 (Cu,Ag)/g–C 3 N 4 –(Cu,Ag)-WO 3 (002) 和 Z 型 (Cu,Ru)/g–C 3 N 4 –(Cu,Ru)-WO 3(002),是通过光辅助静电自组装方法制备的。通过XRD、SEM、TEM、EDX、XPS、DRS、PL、TPR和EIS对制备的光催化剂进行综合表征。评估了不同助催化剂组合对光催化剂活性和选择性的影响。此外,还研究了模拟太阳光照射时间、NO 3 -初始浓度和循环时间对NO 3 -和SO 3 2-转化率的影响。结果表明,Z-scheme (Cu,Ag)/g-C 3 N 4 –(Cu,Ag)-WO 3 (002) 光催化剂可以选择性地将NO 3 -转化为N 2,而Z-方案(Cu,Ru)/g–C 3 N 4 –(Cu,Ru)-WO 3 (002) 光催化剂可以选择性地将NO 3 -转化为NH 4 +。然而,Z-方案(Cu,Cu)/g-C 3 N 4 -(Cu,Cu)-WO 3 (002) 光催化剂对NO 3 - 的转化几乎没有选择性。由于WO 3的高活性(002)面的存在,这三种光催化剂均表现出较强的将SO 3 2-转化为硫酸盐(SO 4 2- )的能力。最后,NO光催化转化的可能机制提出了由 Z 型 (Cu,M)/g-C 3 N 4 –(Cu,M)-WO 3 (002) (M = Cu、Ag 和 Ru) 光催化剂引起的3 -和 SO 3 2-。



























 京公网安备 11010802027423号
京公网安备 11010802027423号