Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-04 , DOI: 10.1016/j.bioorg.2021.105331 Sinthiya J Gawandi 1 , Vidya G Desai 1 , Shrinivas Joshi 2 , Sunil Shingade 3 , Raghuvir R Pissurlenkar 4
|
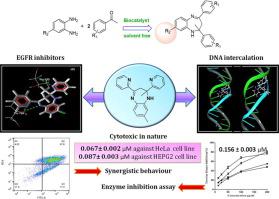
Herein, we designed and synthesized 1,5-benzodiazepines as a lead molecule for anticancer activity and as potent synergistic activity with drug Methotrexate. Working under the framework of green chemistry principles, series of 1,5-benzodiazepine derivatives (3a-3a1) were synthesized using biocatalyst i.e. thiamine hydrochloride under solvent free neat heat conditions. These compounds were screened for in vitro anti cancer activity against couple of cancer cell lines (HeLa and HEPG2) and normal human cell line HEK-293 via MTT assay. The IC50 values for the compounds were in the range 0.067 to 0.35 µM, better than Paclitaxel and compatible with the drug Methotrexate. Compound 3x was found to be influential against both the cell lines with IC50 values of 0.067 ± 0.002 µM against HeLa and 0.087 ± 0.003 µM against HEPG2 cell line, having activity as compatible to the standard drug Methotrexate. Bioinformatic analysis showed that these compounds are good tyrosine kinase inhibitors which was then proved using enzyme inhibition assay. The studies of apoptosis revealed late apoptotic mode of cell death for the compounds against HEPG2 cancer cell line using flow cytometry method. Synergistic studies of compound 3x and drug Methotrexate showed that the combination was highly active against cancer HeLa and HEPG2 cell line with IC50 value 0.046 ± 0.002 µM and 0.057 ± 0.002 µM respectively, which was well supported by apoptosis pathway. Further the compounds proved its scope as DNA intercalating agents, as its molecular docking and DNA binding studies revealed that the compounds would fit well into the DNA strands.
中文翻译:

1,5-苯二氮卓的基本衍生物作为具有协同潜力的抗癌剂的评估
在此,我们设计并合成了 1,5-苯二氮卓类药物作为抗癌活性的先导分子,并与药物甲氨蝶呤具有有效的协同作用。在绿色化学原理的框架下,使用生物催化剂即盐酸硫胺素在无溶剂纯热条件下合成了一系列1,5-苯二氮卓衍生物(3a-3a 1 ) 。通过 MTT 测定法筛选这些化合物对几种癌细胞系(HeLa 和 HEPG2)和正常人细胞系 HEK-293的体外抗癌活性。化合物的 IC 50值在 0.067 至 0.35 µM 范围内,优于紫杉醇,并且与药物甲氨蝶呤相容。复合3x被发现对两种细胞系均有影响,对 HeLa 的 IC 50值为 0.067 ± 0.002 µM,对 HEPG2 细胞系的 IC 50 值为 0.087 ± 0.003 µM,具有与标准药物甲氨蝶呤相容的活性。生物信息学分析表明,这些化合物是良好的酪氨酸激酶抑制剂,随后使用酶抑制试验证明了这一点。使用流式细胞术方法对细胞凋亡的研究揭示了化合物抗 HEPG2 癌细胞系的晚期细胞凋亡模式。化合物3x的协同研究和药物 Methotrexate 表明,该组合对癌症 HeLa 和 HEPG2 细胞系具有高度活性,IC50 值分别为 0.046 ± 0.002 µM 和 0.057 ± 0.002 µM,这得到了细胞凋亡途径的良好支持。此外,这些化合物证明了其作为 DNA 嵌入剂的范围,因为其分子对接和 DNA 结合研究表明,这些化合物可以很好地融入 DNA 链。











































 京公网安备 11010802027423号
京公网安备 11010802027423号