当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Fingolimod Employing Regioselective Aziridine Ring-Opening Reaction as a Key Step
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.oprd.1c00248 Jan Doubský 1 , Stanislav Rádl 1 , Josef Cinibulk 1 , Robert Klvaňa 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.oprd.1c00248 Jan Doubský 1 , Stanislav Rádl 1 , Josef Cinibulk 1 , Robert Klvaňa 1
Affiliation
|
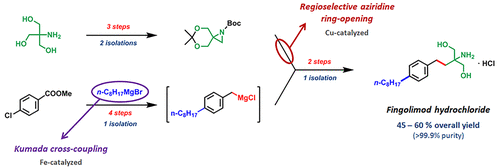
An efficient and scalable synthesis of the immunomodulating drug fingolimod hydrochloride has been developed with the aziridine regioselective ring-opening reaction as a key step. This manuscript describes design, detailed synthetic route scouting, and optimization study of the aziridine ring-opening reaction. As a starting material for the polar part of the fingolimod molecule, cheap, common, and widely commercially available tris(hydroxymethyl)aminomethane was used. n-Octyl group was introduced into the molecule either via Kumada or Negishi cross-couplings, or alternatively by Sonogashira cross-coupling followed by hydrogenation. The final step consists of a one-pot acidic deprotection both of the acetonide and Boc group, providing thus highly pure fingolimod hydrochloride from the crude reaction mixture directly. The described process is highly effective, is industrially applicable, and has been successfully applied to 500 g scales of the target product.
中文翻译:

以区域选择性氮丙啶开环反应为关键步骤合成芬戈莫德
以氮丙啶区域选择性开环反应为关键步骤,开发了一种有效且可扩展的免疫调节药物盐酸芬戈莫德合成方法。这份手稿描述了氮丙啶开环反应的设计、详细的合成路线探索和优化研究。作为芬戈莫德分子极性部分的起始材料,使用廉价、普通和广泛市售的三(羟甲基)氨基甲烷。n-辛基通过 Kumada 或 Negishi 交叉偶联引入分子中,或者通过 Sonogashira 交叉偶联然后氢化。最后一步包括对丙酮化物和 Boc 基团进行一锅酸性脱保护,从而直接从粗反应混合物中提供高纯度的盐酸芬戈莫德。所述工艺高效,具有工业实用性,已成功应用于500g规模的目标产品。
更新日期:2021-09-03
中文翻译:

以区域选择性氮丙啶开环反应为关键步骤合成芬戈莫德
以氮丙啶区域选择性开环反应为关键步骤,开发了一种有效且可扩展的免疫调节药物盐酸芬戈莫德合成方法。这份手稿描述了氮丙啶开环反应的设计、详细的合成路线探索和优化研究。作为芬戈莫德分子极性部分的起始材料,使用廉价、普通和广泛市售的三(羟甲基)氨基甲烷。n-辛基通过 Kumada 或 Negishi 交叉偶联引入分子中,或者通过 Sonogashira 交叉偶联然后氢化。最后一步包括对丙酮化物和 Boc 基团进行一锅酸性脱保护,从而直接从粗反应混合物中提供高纯度的盐酸芬戈莫德。所述工艺高效,具有工业实用性,已成功应用于500g规模的目标产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号