Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2021-09-04 , DOI: 10.1016/j.colsurfb.2021.112098 Ali Altharawi 1 , Sajjad Ahmad 2 , Mubarak A Alamri 1 , Muhammad Tahir Ul Qamar 3
|
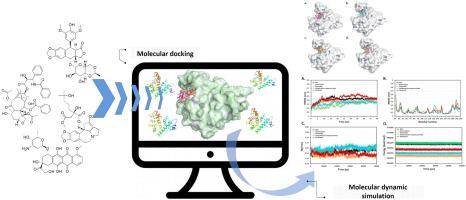
Sorcin (SOluble Resistance-related Calcium bInding proteiN) is a calcium binding protein that plays a key role in multidrug resistance (MDR) in human cancers. This study aimed at understanding the binding mechanism and structural basis for the interaction of structurally and functionally unrelated chemotherapeutic agent, namely doxorubicin, etoposide, omacetaxine mepesuccinate and paclitaxel with Sorcin by utilizing docking and molecular dynamic simulation approaches. The docking evaluation of etoposide, omacetaxine mepesuccinate and paclitaxel have shown a high affinity binding with Sorcin at the Ca2+-binding C-terminal domain (SCBD) in a comparable mode and affinity of binding to doxorubicin. Moreover, all of the docked compounds were shown to interact both hydrophilically and hydrophobically with the same residues within the active pocket which is located at interface of the Sorcin and collectively formed by EF5 loop, G helix and EF4 loop. However, the MD simulations revealed that the dynamics of Sorcin structure is different in the presence of the compounds when compared and contrasted to the Apo Sorcin, particularly in the first 25 ns, after which each system gained considerable structure stability. The difference in dynamics might be the outcome of high N and C-terminal flexibility that seem not to disturb compounds binding conformation but more likely is affecting chemical interaction network by breaking and establishing old and new hydrogen bonds, respectively. This detailed mechanistic understanding of different chemotherapeutic agents binding to Sorcin might be useful to open windows for designing and developing new inhibitors that are potentially capable of reversing the MDR in human cancers.
中文翻译:

通过对接和分子动力学模拟对化疗药物与 Sorcin 的结合模式和相互作用机制的结构洞察
Sorcin(可溶性抗性相关钙结合蛋白)是一种钙结合蛋白,在人类癌症的多药抗性 (MDR) 中起关键作用。本研究旨在通过对接和分子动力学模拟方法了解结构和功能无关的化疗药物,即多柔比星、依托泊苷、奥西他星甲氧基琥珀酸酯和紫杉醇与 Sorcin 相互作用的结合机制和结构基础。依托泊苷、奥美他星甲氧基琥珀酸酯和紫杉醇的对接评估显示在 Ca 2+处与 Sorcin 具有高亲和力结合-结合 C 端结构域 (SCBD) 的可比模式和与阿霉素的结合亲和力。此外,所有对接的化合物都显示出与位于 Sorcin 界面并由 EF5 环、G 螺旋和 EF4 环共同形成的活性口袋内的相同残基进行亲水和疏水相互作用。然而,MD 模拟表明,与 Apo Sorcin 相比,在化合物存在下,Sorcin 结构的动力学是不同的,特别是在前 25 ns,之后每个系统都获得了相当大的结构稳定性。动力学上的差异可能是 N 端和 C 端高度灵活性的结果,它们似乎不会干扰化合物的结合构象,但更有可能通过分别破坏和建立新旧氢键来影响化学相互作用网络。这种对与 Sorcin 结合的不同化学治疗剂的详细机制理解可能有助于打开设计和开发可能能够逆转人类癌症 MDR 的新抑制剂的窗口。











































 京公网安备 11010802027423号
京公网安备 11010802027423号