Journal of Energy Storage ( IF 8.9 ) Pub Date : 2021-09-04 , DOI: 10.1016/j.est.2021.103167 Lei Liu 1 , Zijian Zhou 1 , Changqing Wang 1 , Jie Xu 1 , Hongqiang Xia 2 , Guozhang Chang 2 , Xiaowei Liu 1 , Minghou Xu 1
|
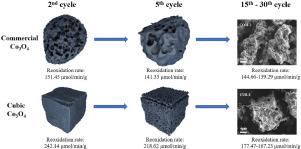
Co3O4 has been regarded as one of the most promising materials for redox energy storage due to its high theoretical conversion and complete redox reversibility. However, when undergoing charge-discharge cycles at high temperature, the material becomes sintered. The reversibility of the cobaltous oxides highly deteriorates, and the reoxidation reaction rate notably decreases. In addition, the obvious thermal hysteresis in the redox reaction process also limits the energy storage performance of the material. In this work, Co3O4 with different micro-nanostructured morphologies is synthesized to evaluate its thermochemical energy storage performance. The capacity of the synthesized and commercial Co3O4 is tested by thermogravimetric analysis (TGA). The fresh and recycled oxides are well characterized. Results suggest that all samples show complete reversibility, and the conversion decreases slightly after 30 cycles. The synthesized samples show better performance, especially cubic Co3O4. The reoxidation rate of the commercial sample is approximately 150 μmol/min/g, but that of the cubic sample is approximately 300-200 μmol/min/g in the first five cycles. The oxidation rate of the cubic sample decreases and finally remains at about 180 μmol/min/g from the 5th cycle to the 30th cycle. After 5 charge-discharge cycles, the commercial Co3O4 is severely sintered, while the cubic oxides are only slightly sintered. After 30 redox cycles, the morphology of commercial oxides completely changed, while that of the cubic oxide retains the approximate shape of a cube. Moreover, the thermal hysteresis values of the commercial and cubic Co3O4 are 24.7°C and 10.1°C, respectively, suggesting that the energy loss of cubic oxides is much smaller. The higher exothermic temperature of the cubic Co3O4 redox process also indicates that it can supply high-grade thermal energy.
中文翻译:

具有立方微纳米结构的 Co3O4/CoO 氧化还原对具有优异的热化学储能性能
Co 3 O 4因其高的理论转化率和完全的氧化还原可逆性而被认为是最有前途的氧化还原储能材料之一。然而,当在高温下进行充放电循环时,材料会发生烧结。氧化钴的可逆性严重恶化,再氧化反应速率显着降低。此外,氧化还原反应过程中明显的热滞现象也限制了材料的储能性能。在这项工作中,合成了具有不同微纳米结构形态的Co 3 O 4以评估其热化学储能性能。合成和商业 Co 3 O的容量图4通过热重分析(TGA)测试。新鲜的和回收的氧化物得到了很好的表征。结果表明,所有样品均表现出完全可逆性,并且在 30 次循环后转化率略有下降。合成的样品表现出更好的性能,尤其是立方Co 3 O 4。商业样品的再氧化速率约为 150 μmol/min/g,而立方体样品在前五个循环中的再氧化速率约为 300-200 μmol/min/g。立方体样品的氧化速率从第 5 次循环到第 30 次循环降低,最终保持在 180 μmol/min/g 左右。5 次充放电循环后,商业 Co 3 O 4严重烧结,而立方氧化物仅轻微烧结。经过 30 次氧化还原循环后,商业氧化物的形态完全改变,而立方氧化物的形态保持近似立方体的形状。此外,商业和立方 Co 3 O 4的热滞后值分别为 24.7°C 和 10.1°C,表明立方氧化物的能量损失要小得多。立方Co 3 O 4氧化还原过程较高的放热温度也表明它可以提供高品位的热能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号