当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glucosyl-1,2,3-triazoles derived from eugenol and analogues: Synthesis, anti-Candida activity, and molecular modeling studies in CYP-51
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-09-04 , DOI: 10.1111/cbdd.13948 Lorena Severiano de Magalhães 1 , Adriana Cotta Cardoso Reis 1 , Izadora Amaral Nakao 1 , Vinícius Augusto Campos Péret 1 , Rúbia Castro Fernandes Melo Reis 1 , Naiara Chaves Silva 2 , Amanda Latércia Tranches Dias 2 , Diogo Teixeira Carvalho 3 , Danielle Ferreira Dias 4 , Geraldo Célio Brandão 1 , Saulo Fehelberg Pinto Braga 1 , Thiago Belarmino de Souza 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-09-04 , DOI: 10.1111/cbdd.13948 Lorena Severiano de Magalhães 1 , Adriana Cotta Cardoso Reis 1 , Izadora Amaral Nakao 1 , Vinícius Augusto Campos Péret 1 , Rúbia Castro Fernandes Melo Reis 1 , Naiara Chaves Silva 2 , Amanda Latércia Tranches Dias 2 , Diogo Teixeira Carvalho 3 , Danielle Ferreira Dias 4 , Geraldo Célio Brandão 1 , Saulo Fehelberg Pinto Braga 1 , Thiago Belarmino de Souza 1
Affiliation
|
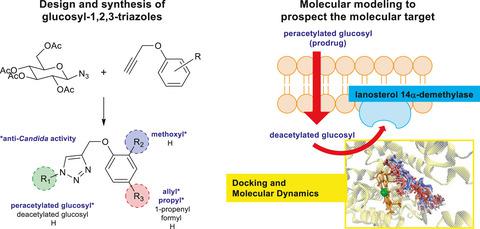
This work describes the synthesis, anti-Candida, and molecular modeling studies of eighteen new glucosyl-1,2,3-triazoles derived from eugenol and correlated phenols. The new compounds were characterized by combined Fourier Transform Infrared, 1H and 13C nuclear magnetic resonance and spectroscopy of high-resolution mass spectrometry. The synthesized compounds did not show significant cytotoxicity against healthy fibroblast human cells (MCR-5) providing interesting selectivity indexes (SI) to active compounds. Considering the antifungal activity, nine compounds showed anti-Candida potential and the peracetylated triazoles 17 and 18 were the most promising ones. Eugenol derivative 17 was active against three species of Candida at 26.1–52.1 μM. This compound was four times more potent than fluconazole against Candida krusei and less toxic (SI > 6.6) against the MCR-5 cells than fluconazole (SI > 3.3) considering this strain. Dihydroeugenol derivative 18 showed similar activity to 17 and was four times more potent and less toxic than fluconazole against C. krusei. The deacetylated glucosides and non-glucosylated corresponding derivatives did not show considerable antifungal action, suggesting that the acetyl groups are essential for their anti-Candida activity. Molecular docking coupled with molecular dynamics showed that 14α-lanosterol demethylase is a feasible molecular target, since 17 and 18 could bind to this enzyme once deacetylated in vivo, thereby acting as prodrugs. Also, these studies demonstrated the importance of hydrophobic substituents at the phenyl ring.
中文翻译:

丁香酚及其类似物衍生的 Glucosyl-1,2,3-triazoles:CYP-51 中的合成、抗念珠菌活性和分子模型研究
这项工作描述了18 种源自丁香酚和相关酚类的新型 glucosyl-1,2,3-triazoles的合成、抗念珠菌和分子建模研究。新化合物通过组合傅里叶变换红外、1 H 和13 C 核磁共振和高分辨率质谱法进行表征。合成的化合物对健康的成纤维细胞人类细胞 (MCR-5) 没有显示出显着的细胞毒性,为活性化合物提供了有趣的选择性指数 (SI)。考虑到抗真菌活性,9 种化合物显示出抗念珠菌的潜力,而过乙酰化三唑 17 和 18 是最有希望的化合物。丁香酚衍生物 17 对三种念珠菌在 26.1–52.1 μM。考虑到该菌株,该化合物对克氏念珠菌的效力是氟康唑的四倍,对 MCR-5 细胞的毒性 (SI > 6.6) 比氟康唑 (SI > 3.3) 低。二氢丁香酚衍生物 18 表现出与 17 相似的活性,并且比氟康唑对C的效力强四倍,毒性更低。 克鲁赛 脱乙酰糖苷和非糖基化的相应衍生物没有显示出显着的抗真菌作用,这表明乙酰基对其抗念珠菌至关重要。活动。分子对接结合分子动力学表明,14α-羊毛甾醇脱甲基酶是一种可行的分子靶点,因为17和18一旦在体内脱乙酰化就可以与该酶结合,从而充当前药。此外,这些研究证明了苯环上疏水取代基的重要性。
更新日期:2021-10-29
中文翻译:

丁香酚及其类似物衍生的 Glucosyl-1,2,3-triazoles:CYP-51 中的合成、抗念珠菌活性和分子模型研究
这项工作描述了18 种源自丁香酚和相关酚类的新型 glucosyl-1,2,3-triazoles的合成、抗念珠菌和分子建模研究。新化合物通过组合傅里叶变换红外、1 H 和13 C 核磁共振和高分辨率质谱法进行表征。合成的化合物对健康的成纤维细胞人类细胞 (MCR-5) 没有显示出显着的细胞毒性,为活性化合物提供了有趣的选择性指数 (SI)。考虑到抗真菌活性,9 种化合物显示出抗念珠菌的潜力,而过乙酰化三唑 17 和 18 是最有希望的化合物。丁香酚衍生物 17 对三种念珠菌在 26.1–52.1 μM。考虑到该菌株,该化合物对克氏念珠菌的效力是氟康唑的四倍,对 MCR-5 细胞的毒性 (SI > 6.6) 比氟康唑 (SI > 3.3) 低。二氢丁香酚衍生物 18 表现出与 17 相似的活性,并且比氟康唑对C的效力强四倍,毒性更低。 克鲁赛 脱乙酰糖苷和非糖基化的相应衍生物没有显示出显着的抗真菌作用,这表明乙酰基对其抗念珠菌至关重要。活动。分子对接结合分子动力学表明,14α-羊毛甾醇脱甲基酶是一种可行的分子靶点,因为17和18一旦在体内脱乙酰化就可以与该酶结合,从而充当前药。此外,这些研究证明了苯环上疏水取代基的重要性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号