当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Does the inability of CA1 area to respond to ischemia with early rapid adenosine release contribute to hippocampal vulnerability?
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-09-03 , DOI: 10.1111/jnc.15498 Natalia V Gulyaeva 1, 2
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-09-03 , DOI: 10.1111/jnc.15498 Natalia V Gulyaeva 1, 2
Affiliation
|
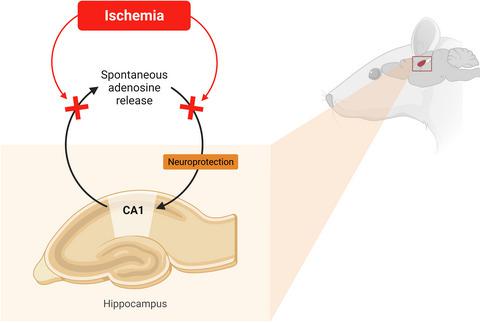
This Editorial highlights a remarkable study in the current issue of the Journal of Neurochemistry in which Ganesana & Venton (2021) report new data showing that brain ischemia does not elicit transient adenosine release in the CA1 hippocampal area. Using fast-scan cyclic voltammetry at a carbon-fiber microelectrode implanted in the CA1 subfield of the hippocampus, it was shown that none of three different ischemia/reperfusion models could increase spontaneous, transient adenosine release, and more severe models even suppressed this presumably neuroprotective release. Since the authors have previously shown that in the caudate putamen, ischemia increased the frequency of spontaneous adenosine release (Ganesana & Venton, 2018), the new data may disclose a mechanism underlying important regional differences in rapid neuroprotective adenosine signaling. The phenomenon of selective susceptibility of the hippocampus to ischemia/hypoxia is well-documented, and the reported failure of its CA1 area to respond to ischemia by rapid adenosine release may be indicative of an insufficiency of this neuroprotective mechanism contributing to hippocampal vulnerability.
中文翻译:

CA1 区域无法通过早期快速腺苷释放来应对缺血是否会导致海马脆弱性?
本社论重点介绍了最新一期《神经化学杂志》上的一项非凡研究,其中 Ganesana & Venton (2021) 报告了新数据,表明脑缺血不会引起 CA1 海马区的瞬时腺苷释放。在植入海马 CA1 亚区的碳纤维微电极上使用快速扫描循环伏安法,结果表明三种不同的缺血/再灌注模型都不能增加自发的、瞬时的腺苷释放,更严重的模型甚至抑制了这种可能具有神经保护作用的发布。由于作者之前已经表明,在尾壳核中,缺血增加了自发腺苷释放的频率(Ganesana & Venton,2018 年),新数据可能揭示了快速神经保护腺苷信号传导的重要区域差异背后的机制。海马体对缺血/缺氧的选择性易感性现象已得到充分证明,据报道其 CA1 区域无法通过快速腺苷释放对缺血做出反应,这可能表明这种神经保护机制不足,导致海马体脆弱。
更新日期:2021-09-03
中文翻译:

CA1 区域无法通过早期快速腺苷释放来应对缺血是否会导致海马脆弱性?
本社论重点介绍了最新一期《神经化学杂志》上的一项非凡研究,其中 Ganesana & Venton (2021) 报告了新数据,表明脑缺血不会引起 CA1 海马区的瞬时腺苷释放。在植入海马 CA1 亚区的碳纤维微电极上使用快速扫描循环伏安法,结果表明三种不同的缺血/再灌注模型都不能增加自发的、瞬时的腺苷释放,更严重的模型甚至抑制了这种可能具有神经保护作用的发布。由于作者之前已经表明,在尾壳核中,缺血增加了自发腺苷释放的频率(Ganesana & Venton,2018 年),新数据可能揭示了快速神经保护腺苷信号传导的重要区域差异背后的机制。海马体对缺血/缺氧的选择性易感性现象已得到充分证明,据报道其 CA1 区域无法通过快速腺苷释放对缺血做出反应,这可能表明这种神经保护机制不足,导致海马体脆弱。



























 京公网安备 11010802027423号
京公网安备 11010802027423号