当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The drebrin/EB3 pathway regulates cytoskeletal dynamics to drive neuritogenesis in embryonic cortical neurons
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-09-03 , DOI: 10.1111/jnc.15502 Thanushiyan Poobalasingam 1 , Francesca Bianco 2 , Fazal Oozeer 3 , Phillip R Gordon-Weeks 3
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-09-03 , DOI: 10.1111/jnc.15502 Thanushiyan Poobalasingam 1 , Francesca Bianco 2 , Fazal Oozeer 3 , Phillip R Gordon-Weeks 3
Affiliation
|
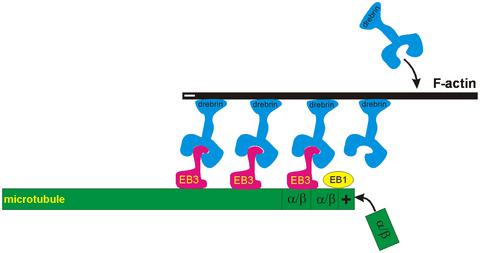
Co-ordinating the dynamic behaviour of actin filaments (F-actin) and microtubules in filopodia is an important underlying process in neuritogenesis, but the molecular pathways involved are ill-defined. The drebrin/end-binding protein 3 (EB3) pathway is a candidate pathway for linking F-actin to microtubules in filopodia. Drebrin binds F-actin and, simultaneously, the microtubule-binding protein EB3 when bound to microtubule plus-ends. We assessed the effect on neuritogenesis of gain- or loss-of-function of proteins in the drebrin/EB3 pathway in rat embryonic cortical neurons in culture. Loss-of-function of drebrin by gene editing or pharmacological inhibition of drebrin binding to F-actin reduced the number of dynamic microtubules in the cell periphery and simultaneously delayed the initiation of neuritogenesis, whereas over-expression of drebrin induced supernumerary neurites. Similarly, loss of EB3 inhibited neuritogenesis, whereas loss of end-binding protein 1 (EB1), a related protein that does not bind to drebrin, did not affect neuritogenesis. Over-expression of EB3, but not EB1, induced supernumerary neurites. We discovered that EB3 is more proximally located at dynamic microtubule plus-ends than EB1 in growth cone filopodia allowing for continuous microtubule elongation as the drebrin/EB3 pathway zippers microtubules to F-actin in filopodia. Finally, we showed that preventing the entry of dynamic microtubules into filopodia using a pharmacological inhibitor of microtubule dynamics is associated with a loss of EB3, but not EB1, from microtubule plus-ends and a concurrent attenuation of neuritogenesis. Collectively, these findings support the idea that neuritogenesis depends on microtubule/F-actin zippering in filopodia orchestrated by the drebrin/EB3 pathway.
中文翻译:

drebrin/EB3 通路调节细胞骨架动力学以驱动胚胎皮质神经元的神经发生
协调丝状伪足中肌动蛋白丝 (F-actin) 和微管的动态行为是神经发生的重要潜在过程,但所涉及的分子途径尚不明确。drebrin/末端结合蛋白 3 (EB3) 途径是将 F-肌动蛋白与丝状伪足中的微管连接的候选途径。当与微管正端结合时,Drebrin 结合 F-肌动蛋白,同时结合微管结合蛋白 EB3。我们评估了培养的大鼠胚胎皮层神经元中drebrin/EB3通路中蛋白质功能获得或丧失对神经发生的影响。通过基因编辑或药理学抑制drebrin与F-肌动蛋白结合的drebrin功能丧失减少了细胞周围动态微管的数量,同时延迟了神经发生的起始,而 drebrin 的过表达诱导了额外的神经突。类似地,EB3 的缺失抑制了神经发生,而末端结合蛋白 1 (EB1)(一种不与 drebrin 结合的相关蛋白)的缺失不影响神经发生。EB3 而非 EB1 的过度表达诱导了额外的神经突。我们发现,在生长锥丝状伪足中,EB3 比 EB1 更靠近动态微管正端,允许连续微管伸长,因为 drebrin/EB3 通路将微管拉到丝状伪足中的 F-肌动蛋白。最后,我们表明,使用微管动力学的药理学抑制剂阻止动态微管进入丝状伪足与微管正端的 EB3 而不是 EB1 的损失以及神经发生的同时减弱有关。集体,
更新日期:2021-09-20
中文翻译:

drebrin/EB3 通路调节细胞骨架动力学以驱动胚胎皮质神经元的神经发生
协调丝状伪足中肌动蛋白丝 (F-actin) 和微管的动态行为是神经发生的重要潜在过程,但所涉及的分子途径尚不明确。drebrin/末端结合蛋白 3 (EB3) 途径是将 F-肌动蛋白与丝状伪足中的微管连接的候选途径。当与微管正端结合时,Drebrin 结合 F-肌动蛋白,同时结合微管结合蛋白 EB3。我们评估了培养的大鼠胚胎皮层神经元中drebrin/EB3通路中蛋白质功能获得或丧失对神经发生的影响。通过基因编辑或药理学抑制drebrin与F-肌动蛋白结合的drebrin功能丧失减少了细胞周围动态微管的数量,同时延迟了神经发生的起始,而 drebrin 的过表达诱导了额外的神经突。类似地,EB3 的缺失抑制了神经发生,而末端结合蛋白 1 (EB1)(一种不与 drebrin 结合的相关蛋白)的缺失不影响神经发生。EB3 而非 EB1 的过度表达诱导了额外的神经突。我们发现,在生长锥丝状伪足中,EB3 比 EB1 更靠近动态微管正端,允许连续微管伸长,因为 drebrin/EB3 通路将微管拉到丝状伪足中的 F-肌动蛋白。最后,我们表明,使用微管动力学的药理学抑制剂阻止动态微管进入丝状伪足与微管正端的 EB3 而不是 EB1 的损失以及神经发生的同时减弱有关。集体,



























 京公网安备 11010802027423号
京公网安备 11010802027423号