当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Shear Rate and Protein Concentration on Amyloidogenesis via Interfacial Shear
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpcb.1c05171 Joe A Adam , Hannah R Middlestead 1 , Nicholas E Debono , Amir H Hirsa
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpcb.1c05171 Joe A Adam , Hannah R Middlestead 1 , Nicholas E Debono , Amir H Hirsa
Affiliation
|
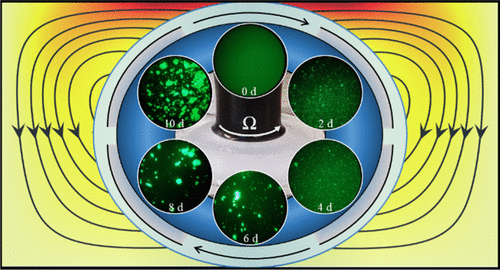
The influence of hydrodynamics on protein fibrillization kinetics is relevant to biophysics, biochemical reactors, medicine, and disease. This investigation focused on the effects of interfacial shear on the fibrillization kinetics of insulin. Human insulin served as a model protein for studying shear-induced fibrillization with relevance to amyloid diseases such as Alzheimer’s, Parkinson’s, prions, and type 2 diabetes. Insulin solutions at different protein concentrations were subjected to shear flows with prescribed interfacial angular velocities using a knife-edge (surface) viscometer (KEV) operating in a laminar axisymmetric flow regime where inertia is significant. Fibrillization kinetics were quantified using intrinsic fibrillization rate and times (onset, half, and end) determined through spectroscopic measurement of monomer extinction curves and fitting to a sigmoidal function. Additionally, the occurrence of gelation was determined through macroscopic imaging and transient fibril microstructure was captured using fluorescence microscopy. The results showed that increasing interfacial shear rate produced a monotonic increase in intrinsic fibrillization rate and a monotonic decrease in fibrillization time. Protein concentration did not significantly impact the intrinsic fibrillization rate or times; however, a minimum fibril concentration for gelation was found. Protein microstructure showed increasing aggregation and plaque/cluster formation with time.
中文翻译:

剪切速率和蛋白质浓度对界面剪切淀粉样变性的影响
流体动力学对蛋白质原纤维化动力学的影响与生物物理学、生化反应器、医学和疾病有关。这项研究的重点是界面剪切对胰岛素原纤维化动力学的影响。人胰岛素作为一种模型蛋白,用于研究与淀粉样疾病(如阿尔茨海默病、帕金森病、朊病毒和 2 型糖尿病)相关的剪切诱导的纤维化。使用刀刃(表面)粘度计 (KEV) 在惯性显着的层流轴对称流态中操作,使不同蛋白质浓度的胰岛素溶液经受具有规定界面角速度的剪切流。使用固有的原纤维化速率和时间(开始、一半、和结束)通过单体消光曲线的光谱测量和拟合到 sigmoidal 函数确定。此外,凝胶化的发生是通过宏观成像确定的,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。通过宏观成像确定凝胶化的发生,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。通过宏观成像确定凝胶化的发生,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。
更新日期:2021-09-16
中文翻译:

剪切速率和蛋白质浓度对界面剪切淀粉样变性的影响
流体动力学对蛋白质原纤维化动力学的影响与生物物理学、生化反应器、医学和疾病有关。这项研究的重点是界面剪切对胰岛素原纤维化动力学的影响。人胰岛素作为一种模型蛋白,用于研究与淀粉样疾病(如阿尔茨海默病、帕金森病、朊病毒和 2 型糖尿病)相关的剪切诱导的纤维化。使用刀刃(表面)粘度计 (KEV) 在惯性显着的层流轴对称流态中操作,使不同蛋白质浓度的胰岛素溶液经受具有规定界面角速度的剪切流。使用固有的原纤维化速率和时间(开始、一半、和结束)通过单体消光曲线的光谱测量和拟合到 sigmoidal 函数确定。此外,凝胶化的发生是通过宏观成像确定的,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。通过宏观成像确定凝胶化的发生,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。通过宏观成像确定凝胶化的发生,并使用荧光显微镜捕获瞬态原纤维微观结构。结果表明,增加界面剪切速率产生固有原纤维化速率的单调增加和原纤维化时间的单调减少。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。蛋白质浓度对固有纤维化率或时间没有显着影响;然而,发现了凝胶化的最小原纤维浓度。蛋白质微观结构显示随着时间的推移聚集和斑块/簇形成增加。









































 京公网安备 11010802027423号
京公网安备 11010802027423号