当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave Study of Triflic Acid Hydrates: Evidence for the Transition from Hydrogen-Bonded Clusters to a Microsolvated Ion Pair
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpca.1c06815 Anna K Huff 1 , Nathan Love 1 , Kenneth R Leopold 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpca.1c06815 Anna K Huff 1 , Nathan Love 1 , Kenneth R Leopold 1
Affiliation
|
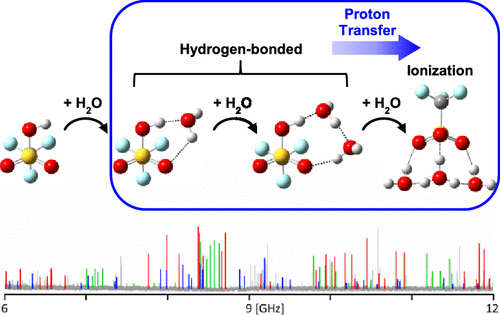
Rotational spectra of the mono-, di-, and trihydrates of triflic acid, CF3SO3H···(H2O)n=1–3, have been recorded by pulsed nozzle Fourier transform microwave spectroscopy and spectroscopic constants obtained have been compared with values calculated at several levels of theory. The experimental results are consistent with the theoretical predictions presented here and elsewhere, indicating that with only one or two water molecules, triflic acid remains un-ionized in a cold molecular complex. The experiments further concur with theoretical predictions that the addition of a third water molecule transforms the system into what is best regarded as a hydrated hydronium triflate ion pair. Thus, only three water molecules are needed to induce ionization of triflic acid in a cold molecular cluster. This number is somewhat low compared with that for other simple protic acids and likely reflects the superacidity of triflic acid. Simple energetic arguments can be used to rationalize this result.
中文翻译:

三氟甲磺酸水合物的微波研究:从氢键簇转变为微溶剂化离子对的证据
三氟甲磺酸的单水合物、二水合物和三水合物的旋转光谱,CF 3 SO 3 H...(H 2 O) n =1–3,已通过脉冲喷嘴傅里叶变换微波光谱记录,并且获得的光谱常数与在几个理论水平计算的值进行了比较。实验结果与这里和其他地方提出的理论预测一致,表明只有一两个水分子,三氟甲磺酸在冷分子复合物中保持未电离。实验进一步与理论预测一致,即添加第三个水分子会将系统转变为最好被视为水合三氟甲磺酸氢铵离子对。因此,仅需要三个水分子来诱导冷分子簇中的三氟甲磺酸电离。与其他简单质子酸相比,这个数字略低,可能反映了三氟甲磺酸的超强酸性。
更新日期:2021-09-16
中文翻译:

三氟甲磺酸水合物的微波研究:从氢键簇转变为微溶剂化离子对的证据
三氟甲磺酸的单水合物、二水合物和三水合物的旋转光谱,CF 3 SO 3 H...(H 2 O) n =1–3,已通过脉冲喷嘴傅里叶变换微波光谱记录,并且获得的光谱常数与在几个理论水平计算的值进行了比较。实验结果与这里和其他地方提出的理论预测一致,表明只有一两个水分子,三氟甲磺酸在冷分子复合物中保持未电离。实验进一步与理论预测一致,即添加第三个水分子会将系统转变为最好被视为水合三氟甲磺酸氢铵离子对。因此,仅需要三个水分子来诱导冷分子簇中的三氟甲磺酸电离。与其他简单质子酸相比,这个数字略低,可能反映了三氟甲磺酸的超强酸性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号