Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.yjmcc.2021.08.012 Maria Papadaki 1 , Theerachat Kampaengsri 1 , Samantha K Barrick 2 , Stuart G Campbell 3 , Dirk von Lewinski 4 , Peter P Rainer 4 , Samantha P Harris 5 , Michael J Greenberg 2 , Jonathan A Kirk 1
|
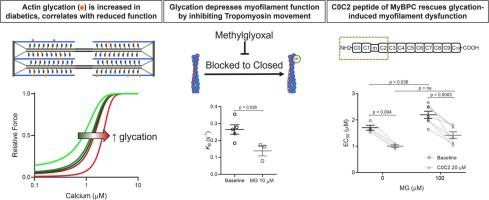
Diabetes doubles the risk of developing heart failure (HF). As the prevalence of diabetes grows, so will HF unless the mechanisms connecting these diseases can be identified. Methylglyoxal (MG) is a glycolysis by-product that forms irreversible modifications on lysine and arginine, called glycation. We previously found that myofilament MG glycation causes sarcomere contractile dysfunction and is increased in patients with diabetes and HF. The aim of this study was to discover the molecular mechanisms by which MG glycation of myofilament proteins cause sarcomere dysfunction and to identify therapeutic avenues to compensate. In humans with type 2 diabetes without HF, we found increased glycation of sarcomeric actin compared to non-diabetics and it correlated with decreased calcium sensitivity. Depressed calcium sensitivity is pathogenic for HF, therefore myofilament glycation represents a promising therapeutic target to inhibit the development of HF in diabetics. To identify possible therapeutic targets, we further defined the molecular actions of myofilament glycation. Skinned myocytes exposed to 100 μM MG exhibited decreased calcium sensitivity, maximal calcium-activated force, and crossbridge kinetics. Replicating MG's functional affects using a computer simulation of sarcomere function predicted simultaneous decreases in tropomyosin's blocked-to-closed rate transition and crossbridge duty cycle were consistent with all experimental findings. Stopped-flow experiments and ATPase activity confirmed MG decreased the blocked-to-closed transition rate. Currently, no therapeutics target tropomyosin, so as proof-of-principal, we used a n-terminal peptide of myosin-binding protein C, previously shown to alter tropomyosin's position on actin. C0C2 completely rescued MG-induced calcium desensitization, suggesting a possible treatment for diabetic HF.
中文翻译:

糖尿病中的肌丝糖化通过抑制原肌球蛋白运动降低收缩力,被 cMyBPC 域拯救
糖尿病会使患心力衰竭 (HF) 的风险增加一倍。随着糖尿病患病率的增加,HF 也会增加,除非可以确定这些疾病之间的关联机制。甲基乙二醛 (MG) 是一种糖酵解副产物,可对赖氨酸和精氨酸形成不可逆修饰,称为糖基化。我们之前发现肌丝 MG 糖化会导致肌节收缩功能障碍,并且在糖尿病和 HF 患者中会增加。本研究的目的是发现肌丝蛋白的 MG 糖化导致肌节功能障碍的分子机制,并确定补偿的治疗途径。在没有 HF 的 2 型糖尿病患者中,我们发现与非糖尿病患者相比,肌节肌动蛋白的糖化增加,并且与钙敏感性降低相关。钙敏感性降低是 HF 的致病因素,因此,肌丝糖化代表了一个很有前途的治疗靶点,可以抑制糖尿病患者 HF 的发展。为了确定可能的治疗靶点,我们进一步定义了肌丝糖化的分子作用。暴露于 100 μM MG 的皮肤肌细胞表现出降低的钙敏感性、最大钙激活力和跨桥动力学。使用肌节功能的计算机模拟复制 MG 的功能影响预测原肌球蛋白的阻断到闭合速率转换和桥占空比的同时减少与所有实验结果一致。停流实验和 ATPase 活性证实 MG 降低了阻塞到关闭的转换率。目前,没有针对原肌球蛋白的疗法,因此作为原理证明,我们使用了肌球蛋白结合蛋白 C 的 n 端肽,先前显示可改变原肌球蛋白在肌动蛋白上的位置。C0C2 完全挽救了 MG 诱导的钙脱敏,表明可能治疗糖尿病性 HF。











































 京公网安备 11010802027423号
京公网安备 11010802027423号