Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.jhep.2021.08.024 Helgi Kristinn Björnsson 1 , Bjorn Gudbjornsson 2 , Einar Stefan Björnsson 3
|
Background & Aims
Infliximab has been associated with drug-induced liver injury (DILI), particularly drug-induced autoimmune hepatitis (DIAIH). DIAIH is commonly treated with corticosteroids, but there is limited data on the efficacy of corticosteroids in infliximab-induced DILI.
Methods
Patients were included for assessment if they had been treated with infliximab between 2009-2020 in Iceland and had developed elevated liver tests. Other specific etiologies of liver enzyme elevations were excluded. Patients treated with corticosteroids were compared to patients not receiving corticosteroids.
Results
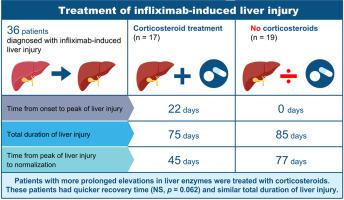
A total of 36 patients with infliximab-induced DILI were identified: median age was 46 years (IQR 32-54) and 28 (78%) were female. Type of liver injury was predominantly hepatocellular (64%). Median peak liver enzymes were: alanine aminotransferase (ALT) 393 (328-695) U/L, aspartate aminotransferase 283 (158-564) U/L, alkaline phosphatase 116 (83-205) U/L, and bilirubin (10-20) 13 μmol/L. A total of 25 (69%) were positive for anti-nuclear antibody and/or had elevated IgG. Corticosteroids were initiated in 17 (47%). Median time from onset of liver injury to peak ALT value was longer in patients treated with corticosteroids, 22 (12-59) vs. 0 (0-3) days (p = 0.001). Time from peak ALT to normalization of liver enzymes was 45 days in the corticosteroid group vs. 77 days in others (p = 0.062). Corticosteroids were tapered in all patients, with no cases of relapse during the follow-up period of 1,245 (820-2,698) days. Overall 75% received another biologic, mostly adalimumab, without evidence of liver injury.
Conclusion
Approximately half of patients with infliximab-induced liver injury had slow improvement in ALT despite cessation of therapy and were treated with corticosteroids. Treatment response was good with prompt resolution of liver test abnormalities. Relapse of liver injury was not observed after tapering of corticosteroids despite prolonged follow-up and no patients developed DILI due to a second biologic.
Lay summary
A rare side effect of infliximab, a biologic medicine used to treat multiple inflammatory diseases, is liver injury and liver inflammation. Steroid treatment has been used in some patients with liver injury caused by infliximab, but there have been few studies supporting this treatment. In this study of 36 patients with infliximab-induced liver injury, approximately half of patients were treated with steroids and the results suggest that patients receiving steroids recover more quickly.
中文翻译:

英夫利昔单抗诱导的肝损伤:临床表型、自身免疫和皮质类固醇治疗的作用
背景与目标
英夫利昔单抗与药物性肝损伤(DILI),尤其是药物性自身免疫性肝炎(DIAIH)有关。DIAIH 通常用皮质类固醇治疗,但关于皮质类固醇在英夫利昔单抗诱导的 DILI 中疗效的数据有限。
方法
如果患者在 2009 年至 2020 年期间在冰岛接受过英夫利昔单抗治疗并且肝功能检查升高,则纳入评估。肝酶升高的其他特定病因被排除在外。将接受皮质类固醇治疗的患者与未接受皮质类固醇治疗的患者进行比较。
结果
共确定了 36 名英夫利昔单抗诱导的 DILI 患者:中位年龄为 46 岁(IQR 32-54),28 名(78%)为女性。肝损伤类型主要是肝细胞(64%)。中位峰值肝酶为:丙氨酸氨基转移酶 (ALT) 393 (328-695) U/L、天冬氨酸氨基转移酶 283 (158-564) U/L、碱性磷酸酶 116 (83-205) U/L 和胆红素 (10- 20) 13 微摩尔/升。共有 25 人(69%)抗核抗体呈阳性和/或 IgG 升高。17 人(47%)开始使用皮质类固醇。接受皮质类固醇治疗的患者从肝损伤发作到 ALT 峰值的中位时间更长,分别为 22 (12-59 )天和0 (0-3) 天 ( p = 0.001)。皮质类固醇组从 ALT 峰值到肝酶正常化的时间为 45 天,而对照组为 45 天。其他 77 天 ( p = 0.062)。所有患者的皮质类固醇逐渐减量,在 1,245 (820-2,698) 天的随访期间没有复发病例。总体而言,75% 接受了另一种生物制剂,主要是阿达木单抗,没有肝损伤的证据。
结论
尽管停止治疗,但大约一半的英夫利昔单抗引起的肝损伤患者的 ALT 改善缓慢,并接受了皮质类固醇治疗。治疗反应良好,肝脏检查异常迅速解决。尽管长期随访,但在逐渐减少皮质类固醇激素后未观察到肝损伤复发,并且没有患者因第二种生物制剂而出现 DILI。
总结
英夫利昔单抗是一种用于治疗多种炎症性疾病的生物药物,其罕见的副作用是肝损伤和肝脏炎症。一些因英夫利昔单抗引起的肝损伤患者已使用类固醇治疗,但支持这种治疗的研究很少。在这项针对 36 名英夫利昔单抗引起的肝损伤患者的研究中,大约一半的患者接受了类固醇治疗,结果表明接受类固醇的患者恢复得更快。











































 京公网安备 11010802027423号
京公网安备 11010802027423号