当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The desalting/salting pathway: a route to form metastable aggregates with tuneable morphologies and lifetimes
Soft Matter ( IF 2.9 ) Pub Date : 2021-08-16 , DOI: 10.1039/d1sm00260k Sumit Mehan 1 , Laure Herrmann 2 , Jean-Paul Chapel 3 , Jacques Jestin 1 , Jean-Francois Berret 2 , Fabrice Cousin 1
Soft Matter ( IF 2.9 ) Pub Date : 2021-08-16 , DOI: 10.1039/d1sm00260k Sumit Mehan 1 , Laure Herrmann 2 , Jean-Paul Chapel 3 , Jacques Jestin 1 , Jean-Francois Berret 2 , Fabrice Cousin 1
Affiliation
|
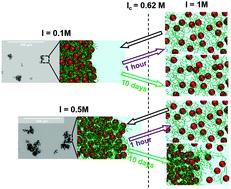
We investigate the formation/re-dissociation mechanisms of hybrid complexes made from negatively charged PAA2k coated γ-Fe2O3 nanoparticles (NP) and positively charged polycations (PDADMAC) in aqueous solution in the regime of very high ionic strength (I). When the building blocks are mixed at large ionic strength (1 M NH4Cl), the electrostatic interaction is screened and complexation does not occur. If the ionic strength is then lowered down to a targeted ionic strength Itarget, there is a critical threshold Ic = 0.62 M at which complexation occurs, that is independent of the charge ratio Z and the pathway used to reduce salinity (drop-by-drop mixing or fast mixing). If salt is added back up to 1 M, the transition is not reversible and persistent out-of-equilibrium aggregates are formed. The lifetimes of such aggregates depends on Itarget: the closer Itarget to Ic is, the more difficult it is to dissolve the aggregates. Such peculiar behavior is driven by the inner structure of the complexes that are formed after desalting. When Itarget is far below Ic, strong electrostatic interactions induce the formation of dense, compact and frozen aggregates. Such aggregates can only poorly reorganize further on with time, which makes their dissolution upon resalting almost reversible. Conversely, when Itarget is close to Ic more open aggregates are formed due to weaker electrostatic interactions upon desalting. The system can thus rearrange with time to lower its free energy and reach more stable out-of-equilibrium states which are very difficult to dissociate back upon resalting, even at very high ionic strength.
中文翻译:

脱盐/盐化途径:形成具有可调形态和寿命的亚稳态聚集体的途径
我们研究了由带负电荷的 PAA 2k包覆的 γ-Fe 2 O 3纳米粒子 (NP) 和带正电荷的聚阳离子 (PDADMAC)制成的杂化复合物在非常高离子强度 (I) 状态下在水溶液中的形成/再离解机制. 当构建块以大离子强度(1 M NH 4 Cl)混合时,静电相互作用被屏蔽并且不会发生络合。如果然后将离子强度降低到目标离子强度I target,则存在一个临界阈值I c = 0.62 M,此时发生络合,这与电荷比Z无关以及用于降低盐度的途径(逐滴混合或快速混合)。如果将盐加回至 1 M,则转变是不可逆的,并且会形成持久的不平衡聚集体。这样的聚集体的寿命取决于我目标:越接近我目标到我Ç就是,越难以溶解的聚集物。这种奇特的行为是由脱盐后形成的复合物的内部结构驱动的。当我的目标远低于我c,强静电相互作用诱导形成致密、致密和冻结的聚集体。随着时间的推移,这种聚集体只能很差地进一步重组,这使得它们在重新盐化时的溶解几乎是可逆的。相反,当I target接近I c 时,由于脱盐时较弱的静电相互作用,会形成更多的开放聚集体。因此,该系统可以随着时间的推移重新排列以降低其自由能并达到更稳定的非平衡状态,即使在非常高的离子强度下,这些状态在重新盐化时也很难解离。
更新日期:2021-09-03
中文翻译:

脱盐/盐化途径:形成具有可调形态和寿命的亚稳态聚集体的途径
我们研究了由带负电荷的 PAA 2k包覆的 γ-Fe 2 O 3纳米粒子 (NP) 和带正电荷的聚阳离子 (PDADMAC)制成的杂化复合物在非常高离子强度 (I) 状态下在水溶液中的形成/再离解机制. 当构建块以大离子强度(1 M NH 4 Cl)混合时,静电相互作用被屏蔽并且不会发生络合。如果然后将离子强度降低到目标离子强度I target,则存在一个临界阈值I c = 0.62 M,此时发生络合,这与电荷比Z无关以及用于降低盐度的途径(逐滴混合或快速混合)。如果将盐加回至 1 M,则转变是不可逆的,并且会形成持久的不平衡聚集体。这样的聚集体的寿命取决于我目标:越接近我目标到我Ç就是,越难以溶解的聚集物。这种奇特的行为是由脱盐后形成的复合物的内部结构驱动的。当我的目标远低于我c,强静电相互作用诱导形成致密、致密和冻结的聚集体。随着时间的推移,这种聚集体只能很差地进一步重组,这使得它们在重新盐化时的溶解几乎是可逆的。相反,当I target接近I c 时,由于脱盐时较弱的静电相互作用,会形成更多的开放聚集体。因此,该系统可以随着时间的推移重新排列以降低其自由能并达到更稳定的非平衡状态,即使在非常高的离子强度下,这些状态在重新盐化时也很难解离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号