当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-catalyzed oxidation of terminal alkynes to glyoxals and their reactions with 2-phenylimidazo[1,2-a]pyridines: one-pot synthesis of 1,2-diones
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ob01507a Shengrong Liao 1, 2, 3 , Huayan Xu 1 , Bin Yang 1 , Junfeng Wang 1, 2 , Xuefeng Zhou 1, 2 , Xiuping Lin 1 , Yonghong Liu 1, 3
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ob01507a Shengrong Liao 1, 2, 3 , Huayan Xu 1 , Bin Yang 1 , Junfeng Wang 1, 2 , Xuefeng Zhou 1, 2 , Xiuping Lin 1 , Yonghong Liu 1, 3
Affiliation
|
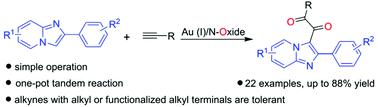
A novel one-pot protocol for the convenient and efficient synthesis of (2-phenylimidazo[1,2-a]pyridin-3-yl)alkane-1,2-diones (3) in good yields (32–88%) from 2-phenylimidazo[1,2-a]pyridines (1) and terminal alkynes (2) has been established with a wide range of substrate scope. A tandem reaction sequence containing gold-catalyzed double oxidations of terminal alkynes to generate glyoxals, nucleophilic addition of 2-phenylimidazo[1,2-a]pyridines to glyoxals to yield α-hydroxyl ketones, and oxygenation of the α-hydroxyl ketones to afford the final products 3 under air atmosphere is involved in this method. Simple operation, mild reaction conditions, and widely available substrates make this strategy more affordable.
中文翻译:

金催化末端炔烃氧化为乙二醛及其与 2-苯基咪唑并[1,2-a]吡啶的反应:一锅法合成 1,2-二酮
一种新颖的一锅法,可方便有效地合成 (2-苯基咪唑并[1,2- a ]吡啶-3-基)烷烃-1,2-二酮 ( 3 ),产率 (32–88%) 2-苯基咪唑并[1,2- a ]吡啶( 1 )和末端炔烃( 2 )已被建立,具有广泛的底物范围。串联反应序列包含金催化末端炔烃的双氧化以生成乙二醛,将 2-苯基咪唑并[1,2- a ]吡啶亲核加成到乙二醛以生成 α-羟基酮,以及将 α-羟基酮氧化以提供最终产品3该方法涉及在空气气氛下。简单的操作、温和的反应条件和广泛可用的底物使这种策略更实惠。
更新日期:2021-09-03
中文翻译:

金催化末端炔烃氧化为乙二醛及其与 2-苯基咪唑并[1,2-a]吡啶的反应:一锅法合成 1,2-二酮
一种新颖的一锅法,可方便有效地合成 (2-苯基咪唑并[1,2- a ]吡啶-3-基)烷烃-1,2-二酮 ( 3 ),产率 (32–88%) 2-苯基咪唑并[1,2- a ]吡啶( 1 )和末端炔烃( 2 )已被建立,具有广泛的底物范围。串联反应序列包含金催化末端炔烃的双氧化以生成乙二醛,将 2-苯基咪唑并[1,2- a ]吡啶亲核加成到乙二醛以生成 α-羟基酮,以及将 α-羟基酮氧化以提供最终产品3该方法涉及在空气气氛下。简单的操作、温和的反应条件和广泛可用的底物使这种策略更实惠。









































 京公网安备 11010802027423号
京公网安备 11010802027423号