当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantification of Conformational Entropy Unravels Effect of Disordered Flanking Region in Coupled Folding and Binding
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-02 , DOI: 10.1021/jacs.1c04214 Frederik Friis Theisen , Lasse Staby , Frederik Grønbæk Tidemand 1 , Charlotte O'Shea , Andreas Prestel , Martin Willemoës , Birthe B Kragelund , Karen Skriver
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-02 , DOI: 10.1021/jacs.1c04214 Frederik Friis Theisen , Lasse Staby , Frederik Grønbæk Tidemand 1 , Charlotte O'Shea , Andreas Prestel , Martin Willemoës , Birthe B Kragelund , Karen Skriver
Affiliation
|
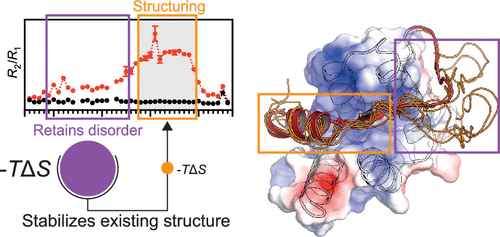
Intrinsic disorder (ID) constitutes a new dimension to the protein structure–function relationship. The ability to undergo conformational changes upon binding is a key property of intrinsically disordered proteins and remains challenging to study using conventional methods. A 1994 paper by R. S. Spolar and M. T. Record presented a thermodynamic approach for estimating changes in conformational entropy based on heat capacity changes, allowing quantification of residues folding upon binding. Here, we adapt the method for studies of intrinsically disordered proteins. We integrate additional data to provide a broader experimental foundation for the underlying relations and, based on >500 protein–protein complexes involving disordered proteins, reassess a key relation between polar and nonpolar surface area changes, previously determined using globular protein folding. We demonstrate the improved suitability of the adapted method to studies of the folded αα-hub domain RST from radical-induced cell death 1, whose interactome is characterized by ID. From extensive thermodynamic data, quantifying the conformational entropy changes upon binding, and comparison to the NMR structure, the adapted method improves accuracy for ID-based studies. Furthermore, we apply the method, in conjunction with NMR, to reveal hitherto undetected effects of interaction–motif context. Thus, inclusion of the disordered context of the DREB2A RST-binding motif induces structuring of the binding motif, resulting in major enthalpy–entropy compensation in the interaction interface. This study, also evaluating additional interactions, demonstrates the strength of the ID-adapted Spolar–Record thermodynamic approach for dissection of structural features of ID-based interactions, easily overlooked in traditional studies, and for translation of these into mechanistic knowledge.
中文翻译:

构象熵的量化揭示了无序侧翼区域在耦合折叠和结合中的影响
内在障碍 (ID) 构成了蛋白质结构-功能关系的新维度。结合后发生构象变化的能力是内在无序蛋白质的一个关键特性,使用传统方法进行研究仍然具有挑战性。RS Spolar 和 MT Record 于 1994 年发表的一篇论文提出了一种热力学方法,用于根据热容量变化来估计构象熵的变化,从而可以量化结合时折叠的残基。在这里,我们调整了研究内在无序蛋白质的方法。我们整合了额外的数据,为潜在的关系提供了更广泛的实验基础,并基于超过 500 个涉及无序蛋白质的蛋白质-蛋白质复合物,重新评估极性和非极性表面积变化之间的关键关系,先前使用球状蛋白质折叠确定。我们证明了改进的方法对研究自由基诱导的细胞死亡 1 的折叠 αα-hub 结构域 RST 的适用性,其相互作用组以 ID 为特征。根据广泛的热力学数据,量化结合后的构象熵变化,并与 NMR 结构进行比较,改进的方法提高了基于 ID 的研究的准确性。此外,我们将该方法与 NMR 结合使用,以揭示迄今为止未检测到的相互作用 - 基序上下文的影响。因此,包含 DREB2A RST 结合基序的无序上下文会诱导结合基序的结构化,导致相互作用界面中的主要焓-熵补偿。这项研究还评估了其他相互作用,
更新日期:2021-09-15
中文翻译:

构象熵的量化揭示了无序侧翼区域在耦合折叠和结合中的影响
内在障碍 (ID) 构成了蛋白质结构-功能关系的新维度。结合后发生构象变化的能力是内在无序蛋白质的一个关键特性,使用传统方法进行研究仍然具有挑战性。RS Spolar 和 MT Record 于 1994 年发表的一篇论文提出了一种热力学方法,用于根据热容量变化来估计构象熵的变化,从而可以量化结合时折叠的残基。在这里,我们调整了研究内在无序蛋白质的方法。我们整合了额外的数据,为潜在的关系提供了更广泛的实验基础,并基于超过 500 个涉及无序蛋白质的蛋白质-蛋白质复合物,重新评估极性和非极性表面积变化之间的关键关系,先前使用球状蛋白质折叠确定。我们证明了改进的方法对研究自由基诱导的细胞死亡 1 的折叠 αα-hub 结构域 RST 的适用性,其相互作用组以 ID 为特征。根据广泛的热力学数据,量化结合后的构象熵变化,并与 NMR 结构进行比较,改进的方法提高了基于 ID 的研究的准确性。此外,我们将该方法与 NMR 结合使用,以揭示迄今为止未检测到的相互作用 - 基序上下文的影响。因此,包含 DREB2A RST 结合基序的无序上下文会诱导结合基序的结构化,导致相互作用界面中的主要焓-熵补偿。这项研究还评估了其他相互作用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号