当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational insights into the membrane fusion mechanism of SARS-CoV-2 at the cellular level
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.csbj.2021.08.053 Jimin Wang 1 , Federica Maschietto 2 , Matthew J Guberman-Pfeffer 2 , Krystle Reiss 2 , Brandon Allen 2 , Yong Xiong 1 , Elias Lolis 3 , Victor S Batista 2
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-09-03 , DOI: 10.1016/j.csbj.2021.08.053 Jimin Wang 1 , Federica Maschietto 2 , Matthew J Guberman-Pfeffer 2 , Krystle Reiss 2 , Brandon Allen 2 , Yong Xiong 1 , Elias Lolis 3 , Victor S Batista 2
Affiliation
|
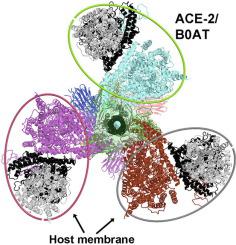
The membrane fusion mechanism of SARS-CoV-2 offers an attractive target for the development of small molecule antiviral inhibitors. Fusion involves an initial binding of the crown-like trimeric spike glycoproteins of SARS-CoV-2 to the receptor angiotensin II-converting enzyme 2 (ACE2) on the permissive host cellular membrane and a prefusion to post-fusion conversion of the spike trimer. During this conversion, the fusion peptides of the spike trimer are inserted into the host membrane to bring together the host and viral membranes for membrane fusion in highly choreographic events. However, geometric constraints due to interactions with the membranes remain poorly understood. In this study, we build structural models of super-complexes of spike trimer/ACE2 dimers based on the molecular structures of the ACE2/neutral amino acid transporter B(0)AT heterodimer. We determine the conformational constraints due to the membrane geometry on the enzymatic activity of ACE2 and on the viral fusion process. Furthermore, we find that binding three ACE2 dimers per spike trimer is essential to open the central pore as necessary for triggering productive membrane fusion through an elongation of the central stalk. The reported findings thus provide valuable insights for targeting the membrane fusion mechanism for drug design at the molecular level.
中文翻译:

细胞水平上 SARS-CoV-2 膜融合机制的计算见解
SARS-CoV-2的膜融合机制为小分子抗病毒抑制剂的开发提供了一个有吸引力的靶点。融合涉及 SARS-CoV-2 的冠状三聚体刺突糖蛋白与允许的宿主细胞膜上的受体血管紧张素 II 转换酶 2 (ACE2) 的初始结合,以及刺突三聚体融合前至融合后的转化。在此转化过程中,刺突三聚体的融合肽被插入宿主膜中,将宿主和病毒膜结合在一起,从而在高度精心设计的事件中实现膜融合。然而,由于与膜相互作用造成的几何限制仍然知之甚少。在本研究中,我们基于 ACE2/中性氨基酸转运蛋白 B(0)AT 异二聚体的分子结构,构建了刺突三聚体/ACE2 二聚体超级复合物的结构模型。我们确定了由于膜几何形状对 ACE2 酶活性和病毒融合过程的构象限制。此外,我们发现每个刺突三聚体结合三个 ACE2 二聚体对于打开中心孔至关重要,这对于通过中心茎的伸长触发有效的膜融合是必要的。因此,报告的发现为分子水平上的药物设计的膜融合机制提供了宝贵的见解。
更新日期:2021-09-03
中文翻译:

细胞水平上 SARS-CoV-2 膜融合机制的计算见解
SARS-CoV-2的膜融合机制为小分子抗病毒抑制剂的开发提供了一个有吸引力的靶点。融合涉及 SARS-CoV-2 的冠状三聚体刺突糖蛋白与允许的宿主细胞膜上的受体血管紧张素 II 转换酶 2 (ACE2) 的初始结合,以及刺突三聚体融合前至融合后的转化。在此转化过程中,刺突三聚体的融合肽被插入宿主膜中,将宿主和病毒膜结合在一起,从而在高度精心设计的事件中实现膜融合。然而,由于与膜相互作用造成的几何限制仍然知之甚少。在本研究中,我们基于 ACE2/中性氨基酸转运蛋白 B(0)AT 异二聚体的分子结构,构建了刺突三聚体/ACE2 二聚体超级复合物的结构模型。我们确定了由于膜几何形状对 ACE2 酶活性和病毒融合过程的构象限制。此外,我们发现每个刺突三聚体结合三个 ACE2 二聚体对于打开中心孔至关重要,这对于通过中心茎的伸长触发有效的膜融合是必要的。因此,报告的发现为分子水平上的药物设计的膜融合机制提供了宝贵的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号