当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Reversibility of Sulfur Reaction with Pt0.3Cu0.7 Nanoparticles
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcc.1c05840 Jocenir Boita 1 , Caio E. A. de Araújo 2 , Lucas Nicolao 1, 2 , Maria do Carmo Martins Alves 3 , Jonder Morais 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcc.1c05840 Jocenir Boita 1 , Caio E. A. de Araújo 2 , Lucas Nicolao 1, 2 , Maria do Carmo Martins Alves 3 , Jonder Morais 1
Affiliation
|
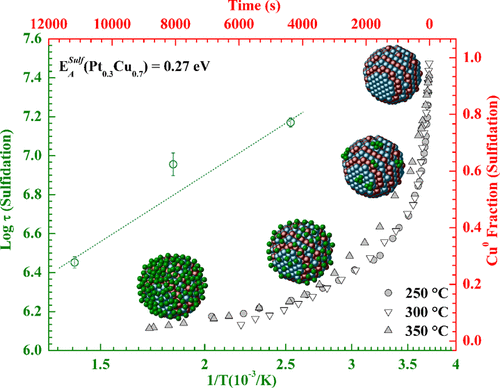
This work unveils the chemical evolution of bimetallic Pt0.3Cu0.7 nanoparticles (NPs) when submitted to oxidizing (H2S) and reductive (H2) atmospheres. Time-resolved X-ray absorption measurements were performed at both Pt-L3 and Cu-K edges in dispersive mode (DXAS), during which near-edge spectra (XANES) were collected sequentially and in situ. These results contributed to the modeling of the Pt0 and Cu0 fraction evolution, from which the activation energies for the sulfidation and reduction processes were estimated. We conclude that the addition of Cu in this bimetallic system improved resistance to sulfur poisoning and its diffusion into the NPs when compared to the Pt-Pd nanoalloys, and moreover allows reversing the S contamination, making it an economically attractive catalyst.
中文翻译:

硫与 Pt0.3Cu0.7 纳米颗粒反应的动力学和可逆性
这项工作揭示了双金属 Pt 0.3 Cu 0.7纳米粒子 (NPs) 在氧化 (H 2 S) 和还原 (H 2 ) 气氛下的化学演变。以色散模式 (DXAS)在 Pt-L 3和 Cu-K 边缘进行时间分辨 X 射线吸收测量,在此期间按顺序原位收集近边缘光谱 (XANES)。这些结果有助于 Pt 0和 Cu 0的建模分数演化,从中估计硫化和还原过程的活化能。我们得出的结论是,与 Pt-Pd 纳米合金相比,在这种双金属系统中添加 Cu 提高了对硫中毒及其扩散到 NPs 的抵抗力,而且还可以逆转 S 污染,使其成为一种经济上有吸引力的催化剂。
更新日期:2021-09-16
中文翻译:

硫与 Pt0.3Cu0.7 纳米颗粒反应的动力学和可逆性
这项工作揭示了双金属 Pt 0.3 Cu 0.7纳米粒子 (NPs) 在氧化 (H 2 S) 和还原 (H 2 ) 气氛下的化学演变。以色散模式 (DXAS)在 Pt-L 3和 Cu-K 边缘进行时间分辨 X 射线吸收测量,在此期间按顺序原位收集近边缘光谱 (XANES)。这些结果有助于 Pt 0和 Cu 0的建模分数演化,从中估计硫化和还原过程的活化能。我们得出的结论是,与 Pt-Pd 纳米合金相比,在这种双金属系统中添加 Cu 提高了对硫中毒及其扩散到 NPs 的抵抗力,而且还可以逆转 S 污染,使其成为一种经济上有吸引力的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号