当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast Transient Infrared Spectroscopy of Photoreceptors with Polarizable QM/MM Dynamics
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpcb.1c05753 Veronica Macaluso 1 , Shaima Hashem 1 , Michele Nottoli 1 , Filippo Lipparini 1 , Lorenzo Cupellini 1 , Benedetta Mennucci 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpcb.1c05753 Veronica Macaluso 1 , Shaima Hashem 1 , Michele Nottoli 1 , Filippo Lipparini 1 , Lorenzo Cupellini 1 , Benedetta Mennucci 1
Affiliation
|
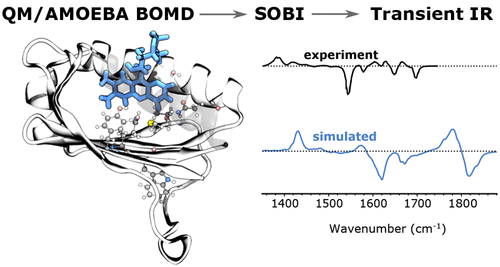
Ultrafast transient infrared (TRIR) spectroscopy is widely used to measure the excitation-induced structural changes of protein-bound chromophores. Here, we design a novel and general strategy to compute TRIR spectra of photoreceptors by combining μs-long MM molecular dynamics with ps-long QM/AMOEBA Born–Oppenheimer molecular dynamics (BOMD) trajectories for both ground and excited electronic states. As a proof of concept, the strategy is here applied to AppA, a blue-light-utilizing flavin (BLUF) protein, found in bacteria. We first analyzed the short-time evolution of the embedded flavin upon excitation revealing that its dynamic Stokes shift is ultrafast and mainly driven by the internal reorganization of the chromophore. A different normal-mode representation was needed to describe ground- and excited-state IR spectra. In this way, we could assign all of the bands observed in the measured transient spectrum. In particular, we could characterize the flavin isoalloxazine-ring region of the spectrum, for which a full and clear description was missing.
中文翻译:

具有可偏振 QM/MM 动力学的光感受器超快瞬态红外光谱
超快瞬态红外(TRIR)光谱广泛用于测量激发引起的蛋白质结合发色团的结构变化。在这里,我们设计了一种新颖且通用的策略,通过将μs长MM分子动力学与ps长QM/AMOEBA Born-Oppenheimer分子动力学(BOMD)轨迹相结合来计算光感受器的TRIR光谱。作为概念证明,该策略应用于 AppA,一种在细菌中发现的蓝光利用黄素 (BLUF) 蛋白。我们首先分析了嵌入黄素在激发时的短时演化,揭示其动态斯托克斯位移超快,并且主要由发色团的内部重组驱动。需要不同的简正模表示来描述基态和激发态红外光谱。通过这种方式,我们可以分配在测量的瞬态频谱中观察到的所有频带。特别是,我们可以表征光谱中黄素异咯嗪环区域的特征,但该区域缺少完整而清晰的描述。
更新日期:2021-09-16
中文翻译:

具有可偏振 QM/MM 动力学的光感受器超快瞬态红外光谱
超快瞬态红外(TRIR)光谱广泛用于测量激发引起的蛋白质结合发色团的结构变化。在这里,我们设计了一种新颖且通用的策略,通过将μs长MM分子动力学与ps长QM/AMOEBA Born-Oppenheimer分子动力学(BOMD)轨迹相结合来计算光感受器的TRIR光谱。作为概念证明,该策略应用于 AppA,一种在细菌中发现的蓝光利用黄素 (BLUF) 蛋白。我们首先分析了嵌入黄素在激发时的短时演化,揭示其动态斯托克斯位移超快,并且主要由发色团的内部重组驱动。需要不同的简正模表示来描述基态和激发态红外光谱。通过这种方式,我们可以分配在测量的瞬态频谱中观察到的所有频带。特别是,我们可以表征光谱中黄素异咯嗪环区域的特征,但该区域缺少完整而清晰的描述。











































 京公网安备 11010802027423号
京公网安备 11010802027423号