当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism Exploration of Potent Fluorinated PI3K Inhibitors with a Triazine Scaffold: Unveiling the Unusual Synergistic Effect of Pyridine-to-Pyrimidine Ring Interconversion and CF3 Defluorination
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcb.1c03242 Abdolkarim Farrokhzadeh 1 , Farideh Badichi Akher 1, 2 , Timothy J Egan 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcb.1c03242 Abdolkarim Farrokhzadeh 1 , Farideh Badichi Akher 1, 2 , Timothy J Egan 1
Affiliation
|
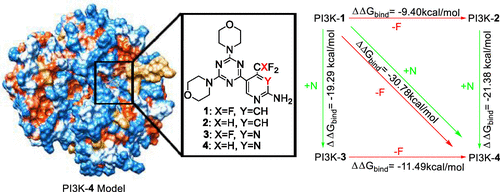
The phosphatidylinostitol-3-kinase (PI3K)/AKT/mammalian target of rapamycin signaling pathway is a vital regulator of cell proliferation, growth, and survival, which is frequently overactivated in many human cancers. To this effect, PI3K, which is an important mediator of this pathway, has been pinpointed as a crucial target in cancer therapy and hence the importance of PI3K inhibitors. It was recently reported that defluorination and pyridine-to-pyrimidine ring interconversion increase the potency of specific small-molecule inhibitors of PI3K. Compound 4, an inhibitor with the difluorinated pyrimidine motif, was found to be eight times more potent against PI3K than compound 1, an inhibitor with the trifluorinated pyridine motif. This observation presents the need to rationally resolve the differential inhibitory mechanisms exhibited by both compounds. In this present work, we employed multiple computational approaches to investigate and distinguish the binding modes of 1 and 4 in addition to the effects they mediate on the secondary structure of PI3K. Likewise, we evaluated two other derivatives, compounds 2 with the difluorinated pyridine motif and 3 with the trifluorinated pyrimidine motif, to investigate the cooperativity effect between the defluorination of CF3 and pyridine-to-pyrimidine ring interconversion. Findings revealed that PI3K, upon interaction with 4, exhibited a series of structural changes that favored the binding of the inhibitor at the active-site region. Furthermore, a positive (synergistic) cooperativity effect was observed between CF3 defluorination and pyridine-to-pyrimidine ring interconversion. Moreover, there was a good correlation between the binding free energy estimated and the biological activity reported experimentally. Energy decomposition analysis revealed that the major contributing force to binding affinity variations between 1 and 4 is the electrostatic energy. Per-residue energy-based hierarchical clustering analysis further identified four hot-spot residues ASP841, TYR867, ASP964, and LYS833 and four warm-spot residues ASP836, SER806, ASP837, and LYS808, which essentially mediate the optimal and higher-affinity binding of compound 4 to PI3K relative to 1. This study therefore provides rational insights into the mechanisms by which 4 exhibited superior PI3K-inhibitory activities over 1, which is vital for future structure-based drug discovery efforts in PI3K targeting.
中文翻译:

具有三嗪支架的强氟化 PI3K 抑制剂的分子机制探索:揭示吡啶-嘧啶环互变和 CF3 脱氟的异常协同效应
雷帕霉素信号通路的磷脂酰肌醇-3-激酶 (PI3K)/AKT/哺乳动物靶点是细胞增殖、生长和存活的重要调节剂,在许多人类癌症中经常被过度激活。为此,PI3K 是该途径的重要介质,已被确定为癌症治疗中的关键靶标,因此 PI3K 抑制剂的重要性。最近有报道称,脱氟和吡啶-嘧啶环互变增加了特定小分子 PI3K 抑制剂的效力。发现化合物4是一种具有二氟化嘧啶基序的抑制剂,其对抗 PI3K 的效力是化合物1 的八倍,一种具有三氟化吡啶基序的抑制剂。这一观察结果表明需要合理解决两种化合物表现出的不同抑制机制。在目前的工作中,除了它们对 PI3K 二级结构的影响外,我们还采用了多种计算方法来研究和区分1和4的结合模式。同样,我们评估了其他两种衍生物,即具有二氟化吡啶基序的化合物2和具有三氟化嘧啶基序的化合物3,以研究 CF 3的脱氟与吡啶环互变之间的协同效应。研究结果表明,PI3K 在与4,表现出一系列有利于抑制剂在活性位点区域结合的结构变化。此外,在CF 3脱氟和吡啶-嘧啶环互变之间观察到正(协同)协同效应。此外,估计的结合自由能与实验报告的生物活性之间存在良好的相关性。能量分解分析表明,导致1和4之间结合亲和力变化的主要因素是静电能。基于每残基能量的层次聚类分析进一步确定了四个热点残基 ASP841、TYR867、ASP964 和 LYS833 和四个热点残基 ASP836、SER806、ASP837 和 LYS808,它们基本上介导了最佳和更高亲和力的结合化合物4相对于1 的PI3K 。因此,该研究提供了合理的见解,通过该机制4表现出比优异的PI3K-抑制活性1,这是至关重要的在PI3K定位未来基于结构的药物发现努力。
更新日期:2021-09-16
中文翻译:

具有三嗪支架的强氟化 PI3K 抑制剂的分子机制探索:揭示吡啶-嘧啶环互变和 CF3 脱氟的异常协同效应
雷帕霉素信号通路的磷脂酰肌醇-3-激酶 (PI3K)/AKT/哺乳动物靶点是细胞增殖、生长和存活的重要调节剂,在许多人类癌症中经常被过度激活。为此,PI3K 是该途径的重要介质,已被确定为癌症治疗中的关键靶标,因此 PI3K 抑制剂的重要性。最近有报道称,脱氟和吡啶-嘧啶环互变增加了特定小分子 PI3K 抑制剂的效力。发现化合物4是一种具有二氟化嘧啶基序的抑制剂,其对抗 PI3K 的效力是化合物1 的八倍,一种具有三氟化吡啶基序的抑制剂。这一观察结果表明需要合理解决两种化合物表现出的不同抑制机制。在目前的工作中,除了它们对 PI3K 二级结构的影响外,我们还采用了多种计算方法来研究和区分1和4的结合模式。同样,我们评估了其他两种衍生物,即具有二氟化吡啶基序的化合物2和具有三氟化嘧啶基序的化合物3,以研究 CF 3的脱氟与吡啶环互变之间的协同效应。研究结果表明,PI3K 在与4,表现出一系列有利于抑制剂在活性位点区域结合的结构变化。此外,在CF 3脱氟和吡啶-嘧啶环互变之间观察到正(协同)协同效应。此外,估计的结合自由能与实验报告的生物活性之间存在良好的相关性。能量分解分析表明,导致1和4之间结合亲和力变化的主要因素是静电能。基于每残基能量的层次聚类分析进一步确定了四个热点残基 ASP841、TYR867、ASP964 和 LYS833 和四个热点残基 ASP836、SER806、ASP837 和 LYS808,它们基本上介导了最佳和更高亲和力的结合化合物4相对于1 的PI3K 。因此,该研究提供了合理的见解,通过该机制4表现出比优异的PI3K-抑制活性1,这是至关重要的在PI3K定位未来基于结构的药物发现努力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号