当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Examining the Transient Dark State in Protein-Quantum Dot Interaction by Relaxation-Based Solution NMR
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcb.1c04853 Muhammed Shafeek Oliyantakath Hassan 1 , Sanoop Mambully Somasundaran 1 , Muhammed Bilal Abdul Shukkoor 1 , Shine Ayyappan 1 , Arshad Abdul Vahid 1 , Vinesh Vijayan 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpcb.1c04853 Muhammed Shafeek Oliyantakath Hassan 1 , Sanoop Mambully Somasundaran 1 , Muhammed Bilal Abdul Shukkoor 1 , Shine Ayyappan 1 , Arshad Abdul Vahid 1 , Vinesh Vijayan 1
Affiliation
|
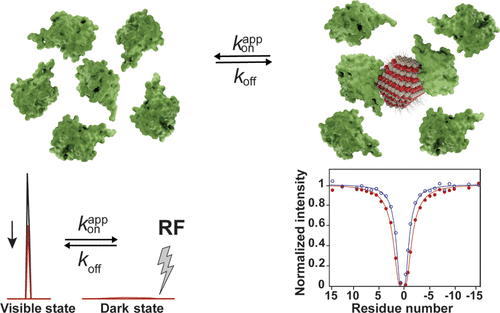
We probed the “dark” state involved in the protein-quantum dot (QD) interaction using a relaxation-based solution nuclear magnetic resonance (NMR) approach. We examined the dynamics and exchange kinetics of the ubiquitin-CdTe model system, which undergoes a fast exchange in the transverse relaxation time scale. We applied the recently developed dark-state exchange saturation transfer (DEST), lifetime line broadening (ΔR2), and exchange-induced chemical shift (δex) solution NMR techniques to obtain a residue-specific binding behavior of the protein on the QD surface. The variation in the estimated 15N–R2bound values clearly shows the dynamic nature of bound Ub. Upon mapping the amino acid residues showing a faster relaxation rate on the electrostatic potential surface of the protein, we have determined that the interaction is preferably electrostatic, and the amino acid residues involved in binding lie on the positively charged surface of the protein. We believe that our experimental approach should provide more in-depth knowledge to engineer new hybrid protein-QD systems in the future.
中文翻译:

通过基于弛豫的溶液核磁共振检查蛋白质-量子点相互作用中的瞬态暗态
我们使用基于弛豫的解决方案核磁共振 (NMR) 方法探测了蛋白质-量子点 (QD) 相互作用中涉及的“暗”状态。我们检查了泛素-CdTe 模型系统的动力学和交换动力学,该系统在横向弛豫时间尺度上经历了快速交换。我们应用最近开发的暗态交换饱和转移 (DEST)、寿命线加宽 (Δ R 2 ) 和交换诱导化学位移 (δ ex ) 溶液 NMR 技术来获得蛋白质在蛋白质上的残基特异性结合行为。量子点表面。估计的15 N– R 2界限的变化值清楚地显示了结合 Ub 的动态特性。在绘制蛋白质静电势表面上显示出更快弛豫速率的氨基酸残基后,我们已经确定相互作用优选地是静电的,并且参与结合的氨基酸残基位于蛋白质的带正电荷的表面上。我们相信我们的实验方法应该提供更深入的知识,以在未来设计新的混合蛋白质 QD 系统。
更新日期:2021-09-16
中文翻译:

通过基于弛豫的溶液核磁共振检查蛋白质-量子点相互作用中的瞬态暗态
我们使用基于弛豫的解决方案核磁共振 (NMR) 方法探测了蛋白质-量子点 (QD) 相互作用中涉及的“暗”状态。我们检查了泛素-CdTe 模型系统的动力学和交换动力学,该系统在横向弛豫时间尺度上经历了快速交换。我们应用最近开发的暗态交换饱和转移 (DEST)、寿命线加宽 (Δ R 2 ) 和交换诱导化学位移 (δ ex ) 溶液 NMR 技术来获得蛋白质在蛋白质上的残基特异性结合行为。量子点表面。估计的15 N– R 2界限的变化值清楚地显示了结合 Ub 的动态特性。在绘制蛋白质静电势表面上显示出更快弛豫速率的氨基酸残基后,我们已经确定相互作用优选地是静电的,并且参与结合的氨基酸残基位于蛋白质的带正电荷的表面上。我们相信我们的实验方法应该提供更深入的知识,以在未来设计新的混合蛋白质 QD 系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号