当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and Dynamic Studies of the F(2P) + ND3 → DF + ND2 Reaction
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpca.1c06515 Rong Xin 1, 2 , Haipan Xiang 1, 2 , Li Tian 1, 2 , Yong Li 2 , Hongwei Song 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.jpca.1c06515 Rong Xin 1, 2 , Haipan Xiang 1, 2 , Li Tian 1, 2 , Yong Li 2 , Hongwei Song 1
Affiliation
|
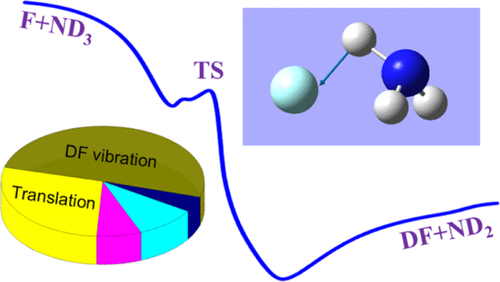
The fast F reaction with NH3 poses a big challenge to experimental studies because of secondary chemical and collisional reactions. The quasi-classical trajectory method is utilized to investigate the mode specificity, product energy disposal, and temperature dependence of the thermal rate coefficient of F + ND3 → DF + ND2 on a recently developed potential energy surface. The effect of isotopic substitution is explored by comparing the F + ND3 reaction with the F + NH3 reaction. The computed results permit a better understanding of the F + ammonia reaction. The DF vibrational state has a Λ-type distribution, in accordance with the experimental measurement by the fast flow reactor technique. The product ND2 is dominantly populated in the ground state, and a considerable amount of ND2 is produced in the fundamental states of the bending mode. The similar vibrational state distributions of HF and NH2 in the F + NH3 reaction indicate a weak isotopic substitution effect on the product energy disposal. Exciting the umbrella mode of ND3 suppresses the reaction at low energies below 5 kcal mol–1, in sharp contrast to the observation in the F + NH3 reaction. These dynamical behaviors can be partially explained by the sudden vector projection model. In addition, the thermal rate coefficient of F + ND3 shows no temperature dependence in the range between 150 and 2000 K. There exists an inverse kinetic isotope effect at temperatures from 150 to 1500 K.
中文翻译:

F(2P) + ND3 → DF + ND2 反应的动力学和动力学研究
由于二次化学和碰撞反应,与NH 3的快速F反应对实验研究提出了巨大挑战。准经典轨迹方法用于研究 F + ND 3 → DF + ND 2在最近开发的势能面上的热速率系数的模式特异性、产物能量处理和温度依赖性。通过比较 F + ND 3反应与 F + NH 3反应来探索同位素取代的影响。计算结果可以更好地理解 F + 氨反应。根据快流反应器技术的实验测量,DF 振动状态具有 Λ 型分布。产品ND2在基态中占主导地位,并且在弯曲模式的基本状态中产生了大量的 ND 2。F + NH 3反应中HF 和NH 2的相似振动态分布表明对产物能量处理的同位素取代作用较弱。激发 ND 3的伞形模式会抑制低于 5 kcal mol –1 的低能量反应,这与 F + NH 3反应中的观察结果形成鲜明对比。这些动态行为可以部分地由突然矢量投影模型来解释。另外,F+ND 3的热率系数 显示在 150 到 2000 K 之间的范围内没有温度依赖性。 在 150 到 1500 K 的温度下存在逆动力学同位素效应。
更新日期:2021-09-16
中文翻译:

F(2P) + ND3 → DF + ND2 反应的动力学和动力学研究
由于二次化学和碰撞反应,与NH 3的快速F反应对实验研究提出了巨大挑战。准经典轨迹方法用于研究 F + ND 3 → DF + ND 2在最近开发的势能面上的热速率系数的模式特异性、产物能量处理和温度依赖性。通过比较 F + ND 3反应与 F + NH 3反应来探索同位素取代的影响。计算结果可以更好地理解 F + 氨反应。根据快流反应器技术的实验测量,DF 振动状态具有 Λ 型分布。产品ND2在基态中占主导地位,并且在弯曲模式的基本状态中产生了大量的 ND 2。F + NH 3反应中HF 和NH 2的相似振动态分布表明对产物能量处理的同位素取代作用较弱。激发 ND 3的伞形模式会抑制低于 5 kcal mol –1 的低能量反应,这与 F + NH 3反应中的观察结果形成鲜明对比。这些动态行为可以部分地由突然矢量投影模型来解释。另外,F+ND 3的热率系数 显示在 150 到 2000 K 之间的范围内没有温度依赖性。 在 150 到 1500 K 的温度下存在逆动力学同位素效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号