当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small Transition-Metal Mixed Clusters as Activators of the C–O Bond. FenCum–CO (n + m = 6): A Theoretical Approach
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpca.1c05919 Patricio Limon 1 , Alan Miralrio 2 , Rodolfo Gómez-Balderas 1 , Miguel Castro 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.jpca.1c05919 Patricio Limon 1 , Alan Miralrio 2 , Rodolfo Gómez-Balderas 1 , Miguel Castro 3
Affiliation
|
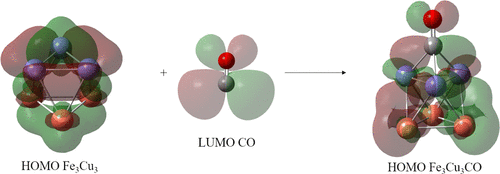
Binding of carbon monoxide, CO, and its activation on the surface of the FenCumCO (n + m = 6) clusters are studied in this work. Using the BPW91/6-311 + G(2d) method, we have found that adsorption of the CO molecule on the surface of FenCum (n + m = 6) clusters is thermochemically favorable. Atop and bridge CO cluster coordinations appear for pure, Fe6 and Cu6, and mixed, Fe2Cu4 and Fe4Cu2, clusters. Threefold coordination takes place for Fe3Cu3–CO where the CO bond length, dCO, suffers a largest increase from 1.128 ± 0.014 Å for bare CO up to 1.21 Å. The CO stretching, νCO, as an indicator for the CO bond weakening is redshifted, from 2099 ± 4 cm–1 for isolated CO up to 1690 cm–1 for Fe3Cu3CO and 1678 cm–1 for Fe6CO. In addition, in Cu6CO, the strongest CO bond is slightly weakened as it has a bond length of 1.15 Å and a νCO of 2029 cm–1. There is a correlation between the CO bond weakening and the increase of CO coordination in FenCumCO, which in turns promotes the transference of charges from the metal core into the antibonding orbitals of CO. Substitution of up to three Cu atoms in Fe6 increases the adsorption energies and the activation of CO. Indeed, FenCum (n + m = 6) are promising clusters to catalyze CO dissociation, particularly Fe3Cu3, Fe5Cu, and Fe6, which have large CO bond lengths and CO adsorption energies. The Bader analysis of the electronic density indicates that FenCumCO species with threefold coordination show a rise in the C–O covalent character due to the less electronic polarization. They also show important M → CO charge transfer, which favors the weakening of the CO bond.
中文翻译:

小的过渡金属混合簇作为 C-O 键的活化剂。FenCum–CO (n + m = 6):理论方法
在这项工作中研究了一氧化碳、CO 的结合及其在 Fe n Cu m CO ( n + m = 6) 簇表面上的活化。使用 BPW91/6-311 + G(2d) 方法,我们发现 CO 分子在 Fe n Cu m ( n + m = 6) 簇表面的吸附在热化学上是有利的。对于纯的 Fe 6和 Cu 6以及混合的 Fe 2 Cu 4和 Fe 4 Cu 2簇,出现顶部和桥接 CO 簇配位。Fe 3 Cu发生三重配位3 -CO,其中 CO 键长d CO从裸 CO 的 1.128 ± 0.014 Å 增加到 1.21 Å。作为 CO 键弱化指标的 CO 拉伸 ν CO发生红移,从孤立 CO 的2099 ± 4 cm –1到Fe 3 Cu 3 CO 的1690 cm –1和Fe 6 CO 的1678 cm –1。此外,在 Cu 6 CO 中,最强的 CO 键略微减弱,因为它的键长为 1.15 Å,ν CO为 2029 cm –1。Fe n Cu 中CO 键减弱与 CO 配位增加之间存在相关性m CO,进而促进电荷从金属核转移到 CO 的反键轨道中。Fe 6中最多三个 Cu 原子的取代增加了吸附能和 CO 的活化。事实上,Fe n Cu m ( n + m = 6) 是催化 CO 解离的有希望的簇,特别是 Fe 3 Cu 3、Fe 5 Cu 和 Fe 6,它们具有较大的 CO 键长和 CO 吸附能。电子密度的 Bader 分析表明 Fe n Cu m由于电子极化较少,具有三重配位的 CO 物种显示出 C-O 共价特性的增加。它们还显示出重要的 M → CO 电荷转移,这有利于 CO 键的减弱。
更新日期:2021-09-16
中文翻译:

小的过渡金属混合簇作为 C-O 键的活化剂。FenCum–CO (n + m = 6):理论方法
在这项工作中研究了一氧化碳、CO 的结合及其在 Fe n Cu m CO ( n + m = 6) 簇表面上的活化。使用 BPW91/6-311 + G(2d) 方法,我们发现 CO 分子在 Fe n Cu m ( n + m = 6) 簇表面的吸附在热化学上是有利的。对于纯的 Fe 6和 Cu 6以及混合的 Fe 2 Cu 4和 Fe 4 Cu 2簇,出现顶部和桥接 CO 簇配位。Fe 3 Cu发生三重配位3 -CO,其中 CO 键长d CO从裸 CO 的 1.128 ± 0.014 Å 增加到 1.21 Å。作为 CO 键弱化指标的 CO 拉伸 ν CO发生红移,从孤立 CO 的2099 ± 4 cm –1到Fe 3 Cu 3 CO 的1690 cm –1和Fe 6 CO 的1678 cm –1。此外,在 Cu 6 CO 中,最强的 CO 键略微减弱,因为它的键长为 1.15 Å,ν CO为 2029 cm –1。Fe n Cu 中CO 键减弱与 CO 配位增加之间存在相关性m CO,进而促进电荷从金属核转移到 CO 的反键轨道中。Fe 6中最多三个 Cu 原子的取代增加了吸附能和 CO 的活化。事实上,Fe n Cu m ( n + m = 6) 是催化 CO 解离的有希望的簇,特别是 Fe 3 Cu 3、Fe 5 Cu 和 Fe 6,它们具有较大的 CO 键长和 CO 吸附能。电子密度的 Bader 分析表明 Fe n Cu m由于电子极化较少,具有三重配位的 CO 物种显示出 C-O 共价特性的增加。它们还显示出重要的 M → CO 电荷转移,这有利于 CO 键的减弱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号