当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Talaromyoxaones A and B: Unusual Oxaphenalenone Spirolactones as Phosphatase Inhibitors from the Marine-Derived Fungus Talaromyces purpureogenus SCSIO 41517
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.joc.1c01452 Xiao Liang 1 , Zhong-Hui Huang 1 , Wen-Bin Shen 2 , Xin-Hua Lu 2 , Xue-Xia Zhang 2 , Xuan Ma 1 , Shu-Hua Qi 1, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.joc.1c01452 Xiao Liang 1 , Zhong-Hui Huang 1 , Wen-Bin Shen 2 , Xin-Hua Lu 2 , Xue-Xia Zhang 2 , Xuan Ma 1 , Shu-Hua Qi 1, 3
Affiliation
|
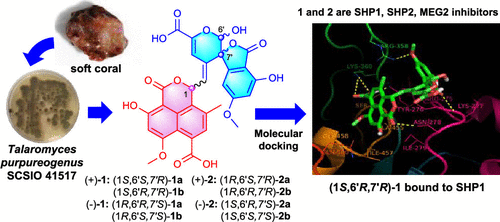
(+)- and (−)-talaromyoxaones A and B (1 and 2, respectively), two new oxaphenalenone derivatives with a hemiacetal frame and an unprecedented spirolactone frame of a 2′H,3H,4′H-spiro[isobenzofuran-1,3′-pyran]-3-one unit that show biosynthetic enantiodivergence, and two new oxaphenalenone analogues (±)-11-apopyrenulin (3) and (+)- or (−)-abeopyrenulin (4) were isolated from the marine-derived fungus Talaromyces purpureogenus SCSIO 41517. Their structures were elucidated by spectroscopic analysis, single-crystal X-ray diffraction, and quantum chemical calculations of ECD spectra. Compounds 1 and 2 showed selective inhibitory activity against phosphatases SHP1, SHP2, and MEG2 with IC50 values of 1.3–3.4 μM, and the potential modes of action for 1 were investigated by a preliminary molecular docking study.
中文翻译:

Talaromyoxaones A 和 B:作为来自海洋来源的真菌 Talaromyces purpureogenus SCSIO 41517 的磷酸酶抑制剂的不寻常 Oxaphenalenone 螺内酯
(+)- 和 (-)-talaromyoxaones A 和 B(分别为1和2),两种具有半缩醛框架的新奥沙芬酮衍生物和前所未有的 2' H ,3 H ,4' H -螺[异苯并呋喃的螺内酯框架-1,3'-吡喃]-3-1 单位显示生物合成对映发散,和两个新的奥沙菲纳酮类似物 (±)-11-apopyrenulin ( 3 ) 和 (+)- 或 (-)-abeopyrenulin ( 4 ) 从海洋来源的真菌Talaromyces purpureogenus SCSIO 41517。通过光谱分析、单晶 X 射线衍射和 ECD 光谱的量子化学计算阐明了它们的结构。化合物1和2显示出对磷酸酶 SHP1、SHP2 和 MEG2 的选择性抑制活性,IC 50值为 1.3-3.4 μM,并且通过初步分子对接研究调查了1的潜在作用模式。
更新日期:2021-09-17
中文翻译:

Talaromyoxaones A 和 B:作为来自海洋来源的真菌 Talaromyces purpureogenus SCSIO 41517 的磷酸酶抑制剂的不寻常 Oxaphenalenone 螺内酯
(+)- 和 (-)-talaromyoxaones A 和 B(分别为1和2),两种具有半缩醛框架的新奥沙芬酮衍生物和前所未有的 2' H ,3 H ,4' H -螺[异苯并呋喃的螺内酯框架-1,3'-吡喃]-3-1 单位显示生物合成对映发散,和两个新的奥沙菲纳酮类似物 (±)-11-apopyrenulin ( 3 ) 和 (+)- 或 (-)-abeopyrenulin ( 4 ) 从海洋来源的真菌Talaromyces purpureogenus SCSIO 41517。通过光谱分析、单晶 X 射线衍射和 ECD 光谱的量子化学计算阐明了它们的结构。化合物1和2显示出对磷酸酶 SHP1、SHP2 和 MEG2 的选择性抑制活性,IC 50值为 1.3-3.4 μM,并且通过初步分子对接研究调查了1的潜在作用模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号