当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Induced Deaminative Alkylation/Cyclization of Alkyl Amines with N-Methacryloyl-2-phenylbenzoimidazoles in Continuous-Flow Organo-Photocatalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.joc.1c01555 Vankudoth Ramesh 1, 2 , Maram Gangadhar 1, 2 , Jagadeesh Babu Nanubolu 2, 3 , Praveen Reddy Adiyala 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.joc.1c01555 Vankudoth Ramesh 1, 2 , Maram Gangadhar 1, 2 , Jagadeesh Babu Nanubolu 2, 3 , Praveen Reddy Adiyala 1, 2
Affiliation
|
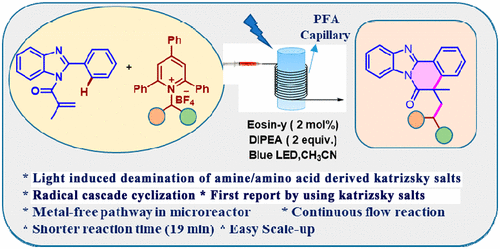
Herein, we present a metal-free visible-light-induced eosin-y-catalyzed deaminative strategy for the sequential alkylation/cyclization of N-methacryloyl-2-phenylbenzoimidazoles with alkyl amine-derived Katritzky salts, which provides an efficient avenue for the construction of various benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-one derivatives in moderate to excellent yields under mild reaction conditions. The key enabling feature of this novel reaction includes utilization of redox-active pyridinium salts from abundant and inexpensive primary amine feedstocks that were converted into alkyl radicals via C–N bond scission and subsequent alkylation/cyclization with N-methacryloyl-2-phenylbenzoimidazoles by the formation of two new C–C bonds. In addition, we implemented this protocol for a variety of amino acids, affording the products in moderate yields. Moreover, the novel, environmentally benign batch protocol was further carried out in a continuous-flow regime by utilizing a perfluoroalkoxy alkane tubing microreactor under optimized reaction conditions with a blue light-emitting diode light source, enabling excellent yields and a shorter reaction time (19 min) versus the long reaction time (16 h) of the batch reaction. The reaction displays excellent functional group tolerance, easy operation, scalability, mild reaction conditions, and broad synthetic utility.
中文翻译:

可见光诱导烷基胺与 N-甲基丙烯酰基-2-苯基苯并咪唑在连续流有机光催化中的脱氨基烷基化/环化
在此,我们提出了一种无金属可见光诱导的曙红-y 催化脱氨基策略,用于N-甲基丙烯酰基-2-苯基苯并咪唑与烷基胺衍生的卡特里茨基盐的顺序烷基化/环化,这为构建提供了有效途径各种苯并[4,5]咪唑并[2,1 - a ]异喹啉-6(5 H )-one衍生物在温和反应条件下以中等至优异的产率合成。这种新颖的反应的关键使能特征包括从丰富且廉价的伯胺原料即分别通过C-N键断裂,并随后烷基化/环化转化为烷基基团的氧化还原活性吡啶鎓盐的利用率Ñ-甲基丙烯酰基-2-苯基苯并咪唑通过形成两个新的 C-C 键。此外,我们对各种氨基酸实施了该协议,以中等产量提供产品。此外,通过在优化的反应条件下使用全氟烷氧基烷烃管式微反应器和蓝色发光二极管光源,在连续流动状态下进一步实施了新的、环境友好的批处理方案,从而实现了优异的产率和更短的反应时间(19 min) 与分批反应的长反应时间 (16 h)。该反应显示出优异的官能团耐受性、易操作性、可扩展性、反应条件温和和广泛的合成用途。
更新日期:2021-09-17
中文翻译:

可见光诱导烷基胺与 N-甲基丙烯酰基-2-苯基苯并咪唑在连续流有机光催化中的脱氨基烷基化/环化
在此,我们提出了一种无金属可见光诱导的曙红-y 催化脱氨基策略,用于N-甲基丙烯酰基-2-苯基苯并咪唑与烷基胺衍生的卡特里茨基盐的顺序烷基化/环化,这为构建提供了有效途径各种苯并[4,5]咪唑并[2,1 - a ]异喹啉-6(5 H )-one衍生物在温和反应条件下以中等至优异的产率合成。这种新颖的反应的关键使能特征包括从丰富且廉价的伯胺原料即分别通过C-N键断裂,并随后烷基化/环化转化为烷基基团的氧化还原活性吡啶鎓盐的利用率Ñ-甲基丙烯酰基-2-苯基苯并咪唑通过形成两个新的 C-C 键。此外,我们对各种氨基酸实施了该协议,以中等产量提供产品。此外,通过在优化的反应条件下使用全氟烷氧基烷烃管式微反应器和蓝色发光二极管光源,在连续流动状态下进一步实施了新的、环境友好的批处理方案,从而实现了优异的产率和更短的反应时间(19 min) 与分批反应的长反应时间 (16 h)。该反应显示出优异的官能团耐受性、易操作性、可扩展性、反应条件温和和广泛的合成用途。










































 京公网安备 11010802027423号
京公网安备 11010802027423号