当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative β-Cleavage of Fused Cyclobutanols Leading to Hydrofuran-Fused Polycyclic Aromatic Compounds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.joc.1c01108 Hayate Kinouchi 1 , Kazuma Sugimoto 1 , Yousuke Yamaoka 1 , Hiroshi Takikawa 1 , Kiyosei Takasu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.joc.1c01108 Hayate Kinouchi 1 , Kazuma Sugimoto 1 , Yousuke Yamaoka 1 , Hiroshi Takikawa 1 , Kiyosei Takasu 1
Affiliation
|
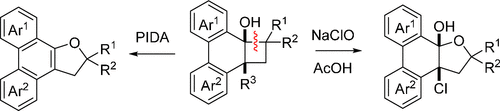
Treatment of aryl-fused bicyclo[4.2.0]octanols with an oxidant such as phenyliodine diacetate (PIDA) or hypochlorous acid gave dihydrofuran-containing polycyclic aromatic compounds by selective β-cleavage of the cyclobutanol moiety. Mechanistic studies suggest that the oxygen atom of the hydrofuran ring is incorporated from the hydroxy group of the substrate via intramolecular addition. The oxidative transformation should serve as a new method to prepare functionalized polycyclic aromatic compounds.
中文翻译:

稠合环丁醇的氧化β-裂解导致氢呋喃稠合多环芳族化合物
用氧化剂如苯碘二乙酸酯 (PIDA) 或次氯酸处理芳基稠合的双环 [4.2.0] 辛醇,通过环丁醇部分的选择性 β-裂解得到含二氢呋喃的多环芳族化合物。机理研究表明,氢呋喃环的氧原子是通过分子内加成从底物的羟基中引入的。氧化转化应作为制备功能化多环芳族化合物的新方法。
更新日期:2021-09-17
中文翻译:

稠合环丁醇的氧化β-裂解导致氢呋喃稠合多环芳族化合物
用氧化剂如苯碘二乙酸酯 (PIDA) 或次氯酸处理芳基稠合的双环 [4.2.0] 辛醇,通过环丁醇部分的选择性 β-裂解得到含二氢呋喃的多环芳族化合物。机理研究表明,氢呋喃环的氧原子是通过分子内加成从底物的羟基中引入的。氧化转化应作为制备功能化多环芳族化合物的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号