当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of Biocatalytic Reductive Amination for the Synthesis of a Key Intermediate to a CDK 2/4/6 Inhibitor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.oprd.1c00255 Shengquan Duan 1 , Daniel W. Widlicka 1 , Michael P. Burns 1 , Rajesh Kumar 1 , Ian Hotham 1 , Jean-Nicolas Desrosiers 1 , Paul Bowles 1 , Kris N. Jones 1 , Lindsay D. Nicholson 1 , Michele T. Buetti-Weekly 1 , Lu Han 1 , Jeremy Steflik 1 , Eric Hansen 1 , Cheryl M. Hayward 1 , Holly Strohmeyer 1 , Sébastien Monfette 1 , Scott C. Sutton 1 , Christopher Morris 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-09-03 , DOI: 10.1021/acs.oprd.1c00255 Shengquan Duan 1 , Daniel W. Widlicka 1 , Michael P. Burns 1 , Rajesh Kumar 1 , Ian Hotham 1 , Jean-Nicolas Desrosiers 1 , Paul Bowles 1 , Kris N. Jones 1 , Lindsay D. Nicholson 1 , Michele T. Buetti-Weekly 1 , Lu Han 1 , Jeremy Steflik 1 , Eric Hansen 1 , Cheryl M. Hayward 1 , Holly Strohmeyer 1 , Sébastien Monfette 1 , Scott C. Sutton 1 , Christopher Morris 1
Affiliation
|
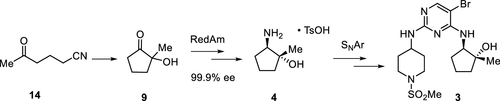
Biocatalytic reductive amination catalyzed by engineered imine reductase (RedAms) is a new and powerful tool for the synthesis of substituted chiral amines. Herein, we describe a streamlined synthesis of compound 3, a key intermediate to a CDK 2/4/6 inhibitor 1, relying on the enzymatic reductive amination of a hydroxyketone to introduce the chiral secondary amine with high diastereoselectivity. The improved synthesis of the hydroxyketone precursor by a titanium-catalyzed reductive cyclization and the process development for two SNAr reactions en route to 3 are also presented.
中文翻译:

生物催化还原胺化在 CDK 2/4/6 抑制剂关键中间体合成中的应用
工程亚胺还原酶(RedAms)催化的生物催化还原胺化是合成取代手性胺的一种新的强大工具。在此,我们描述了化合物 3 的流线型合成,化合物3是 CDK 2/4/6 抑制剂1的关键中间体,依靠羟基酮的酶还原胺化来引入具有高非对映选择性的手性仲胺。还介绍了通过钛催化还原环化改进的羟基酮前体合成和两个 S N Ar 反应过程中的工艺开发3。
更新日期:2021-09-03
中文翻译:

生物催化还原胺化在 CDK 2/4/6 抑制剂关键中间体合成中的应用
工程亚胺还原酶(RedAms)催化的生物催化还原胺化是合成取代手性胺的一种新的强大工具。在此,我们描述了化合物 3 的流线型合成,化合物3是 CDK 2/4/6 抑制剂1的关键中间体,依靠羟基酮的酶还原胺化来引入具有高非对映选择性的手性仲胺。还介绍了通过钛催化还原环化改进的羟基酮前体合成和两个 S N Ar 反应过程中的工艺开发3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号