Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric syntheses of four stereoisomers of 13-hydroxy-14-methylhexadecanoic acid as potential antibacterial agents
Chirality ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1002/chir.23352 Lifeng Wang 1 , Yun Zhou 1 , Xueyang Wang 1 , Gucheng Yuan 1 , Chaonan Yuan 1 , Yuxiong Yang 1 , Qinghua Bian 1 , Min Wang 1 , Jiangchun Zhong 1
Chirality ( IF 2.8 ) Pub Date : 2021-09-03 , DOI: 10.1002/chir.23352 Lifeng Wang 1 , Yun Zhou 1 , Xueyang Wang 1 , Gucheng Yuan 1 , Chaonan Yuan 1 , Yuxiong Yang 1 , Qinghua Bian 1 , Min Wang 1 , Jiangchun Zhong 1
Affiliation
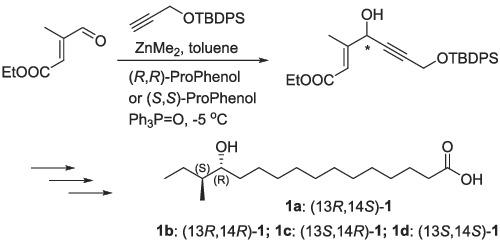
|
The first total syntheses of four stereoisomers of 13-hydroxy-14-methylhexadecanoic acid have been accomplished. Central to this strategy are asymmetric alkynylation of aldehyde, acid-catalyzed lactonization, the selective protection of primary alcohol and Wittig reaction. The product 1a was obtained in 17 steps in 2% overall yield. Moreover, these synthetic chiral hydroxy fatty acids 1a-1d are valuable for the development of antibacterial agents.
中文翻译:

13-羟基-14-甲基十六烷酸四种立体异构体的不对称合成作为潜在的抗菌剂
已经完成了 13-羟基-14-甲基十六烷酸的四种立体异构体的首次全合成。该策略的核心是醛的不对称炔基化、酸催化的内酯化、伯醇的选择性保护和 Wittig 反应。产物1a以 2% 的总产率分 17 步获得。此外,这些合成的手性羟基脂肪酸1a - 1d对于抗菌剂的开发具有重要意义。
更新日期:2021-10-12
中文翻译:

13-羟基-14-甲基十六烷酸四种立体异构体的不对称合成作为潜在的抗菌剂
已经完成了 13-羟基-14-甲基十六烷酸的四种立体异构体的首次全合成。该策略的核心是醛的不对称炔基化、酸催化的内酯化、伯醇的选择性保护和 Wittig 反应。产物1a以 2% 的总产率分 17 步获得。此外,这些合成的手性羟基脂肪酸1a - 1d对于抗菌剂的开发具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号