当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Properties of the H2O–CO2–Na2CO3/CH3OH/NH3 Systems at Low Temperatures and Pressures up to 50 MPa
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acsearthspacechem.1c00066 Victoria Muñoz-Iglesias 1 , Olga Prieto-Ballesteros 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acsearthspacechem.1c00066 Victoria Muñoz-Iglesias 1 , Olga Prieto-Ballesteros 1
Affiliation
|
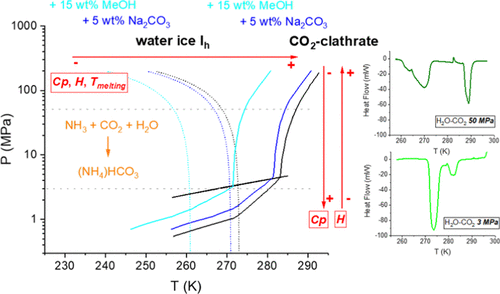
Experimental data on the thermal properties of aqueous solutions at low temperatures and high pressures are necessary to perform an accurate geochemical modeling of ocean worlds, that is, those planetary bodies that can sustain significant reservoirs of the interior liquid for long time periods. We used differential scanning calorimetry (DSC) to determine the values of specific heat capacity and enthalpy of dissociation of CO2-clathrates in the presence of sodium carbonate, ammonia, and methanol at temperatures ≥233 K and pressures ≤50 MPa. We monitored the physicochemical evolution of the systems along the pressure–temperature paths through Raman spectroscopy. The protocol used to form the CO2-clathrates influenced the thermal behavior of the system. Partial recrystallization events from ice to CO2-clathrate during slow heating resulted in out-of-equilibrium heat capacities (Cp) of < 1 J g–1 K–1. After complete melting of the solids, the final aqueous solutions had Cp 2–3 J g–1 K–1. Final fluids richer in dissolved CO2 had a lower Cp. The addition of carbonates and methanol led to a decrease in the melting temperature of both ice and clathrates. Ammonia reacted with CO2 to rapidly form ammonium bicarbonate; however, ice and CO2-clathrates could also stabilize in parallel. In all the studied systems, the formation of clathrates from aqueous solutions without the application of clathrate stabilizers, mechanical agitation, or synthesis from crushed ice led to a low guest occupancy, forming less-stable clathrates with low enthalpies of dissociation (130–275 J g–1). These results have important planetary implications related to the thermal behavior of bodies rich in the compounds of the systems studied, as can be the case of Enceladus. In this research, we show that the level of cage occupancy in clathrates plays a key role in their thermal behavior. Highly occupied clathrates will be robust and will contribute to the retention of heat inside the planetary bodies due to their characteristic low thermal conductivity. However, poorly occupied clathrates will dissociate more easily than water ice, allowing greater heat fluxes through the icy crust and favoring the dissipation of heat to the exterior.
中文翻译:

H2O-CO2-Na2CO3/CH3OH/NH3 系统在低温和高达 50 MPa 的压力下的热性能
在低温和高压下水溶液的热特性的实验数据对于对海洋世界进行准确的地球化学建模是必要的,即那些可以长时间维持大量内部液体的行星体。我们使用差示扫描量热法 (DSC) 来确定在碳酸钠、氨和甲醇存在下,温度 ≥ 233 K 和压力 ≤ 50 MPa时 CO 2包合物的比热容和解离焓值。我们通过拉曼光谱监测了系统沿压力-温度路径的物理化学演变。用于形成 CO 2的协议-包合物影响系统的热行为。在缓慢加热期间从冰到 CO 2包合物的部分重结晶事件导致了< 1 J g –1 K –1 的失衡热容量 ( C p ) 。固体完全熔化后,最终水溶液的C p 2–3 J g –1 K –1。富含溶解 CO 2 的最终流体具有较低的C p。碳酸盐和甲醇的加入导致冰和包合物的熔化温度降低。氨与CO 2反应迅速生成碳酸氢铵;然而,冰和 CO2-包合物也可以同时稳定。在所有研究的系统中,在没有使用包合物稳定剂、机械搅拌或从碎冰合成的情况下从水溶液形成包合物导致客体占有率低,形成具有低解离焓的不太稳定的包合物(130-275 J g –1)。这些结果对富含所研究系统化合物的物体的热行为具有重要的行星意义,土卫二的情况就是如此。在这项研究中,我们表明包合物中笼子的占用水平在其热行为中起着关键作用。高度占据的包合物将是坚固的,并且由于其特有的低导热性将有助于在行星体内保持热量。然而,占据不良的包合物比水冰更容易解离,允许更大的热通量通过冰壳并有利于热量散发到外部。
更新日期:2021-10-22
中文翻译:

H2O-CO2-Na2CO3/CH3OH/NH3 系统在低温和高达 50 MPa 的压力下的热性能
在低温和高压下水溶液的热特性的实验数据对于对海洋世界进行准确的地球化学建模是必要的,即那些可以长时间维持大量内部液体的行星体。我们使用差示扫描量热法 (DSC) 来确定在碳酸钠、氨和甲醇存在下,温度 ≥ 233 K 和压力 ≤ 50 MPa时 CO 2包合物的比热容和解离焓值。我们通过拉曼光谱监测了系统沿压力-温度路径的物理化学演变。用于形成 CO 2的协议-包合物影响系统的热行为。在缓慢加热期间从冰到 CO 2包合物的部分重结晶事件导致了< 1 J g –1 K –1 的失衡热容量 ( C p ) 。固体完全熔化后,最终水溶液的C p 2–3 J g –1 K –1。富含溶解 CO 2 的最终流体具有较低的C p。碳酸盐和甲醇的加入导致冰和包合物的熔化温度降低。氨与CO 2反应迅速生成碳酸氢铵;然而,冰和 CO2-包合物也可以同时稳定。在所有研究的系统中,在没有使用包合物稳定剂、机械搅拌或从碎冰合成的情况下从水溶液形成包合物导致客体占有率低,形成具有低解离焓的不太稳定的包合物(130-275 J g –1)。这些结果对富含所研究系统化合物的物体的热行为具有重要的行星意义,土卫二的情况就是如此。在这项研究中,我们表明包合物中笼子的占用水平在其热行为中起着关键作用。高度占据的包合物将是坚固的,并且由于其特有的低导热性将有助于在行星体内保持热量。然而,占据不良的包合物比水冰更容易解离,允许更大的热通量通过冰壳并有利于热量散发到外部。











































 京公网安备 11010802027423号
京公网安备 11010802027423号