当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Stabilizers on the Performance of Au/TiO2 Catalysts for CO Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c02820 Nating Yang 1, 2 , Samuel Pattisson 1 , Mark Douthwaite 1 , Gaofeng Zeng 2 , Hao Zhang 3 , Jingyuan Ma 4 , Graham J. Hutchings 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c02820 Nating Yang 1, 2 , Samuel Pattisson 1 , Mark Douthwaite 1 , Gaofeng Zeng 2 , Hao Zhang 3 , Jingyuan Ma 4 , Graham J. Hutchings 1
Affiliation
|
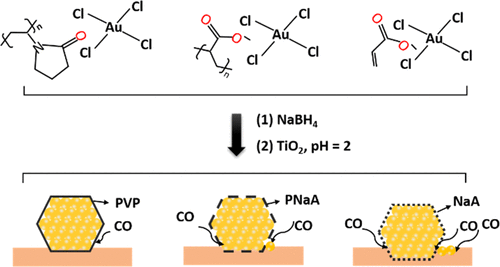
Stabilizers are commonly employed to synthesize nanocrystals with well-defined morphologies and size distributions, making them ideal tools to study structure–activity relationships in heterogeneous catalysts. While it is well documented that stabilizers can influence both the structure and size of the nanocrystals, little emphasis has been placed on how the properties of these species influence catalytic performance. Herein, different polymers (poly-sodium acrylate (PNaA), poly(vinyl alcohol) (PVA), and poly(vinyl pyrrolidone) (PVP)) and the monomer sodium acrylate (NaA) were used as stabilizers for the synthesis of Au nanoparticles supported on TiO2. The mean Au particle size in all of the catalysts was comparable regardless of the stabilizer used; however, the activity of these catalysts toward CO oxidation differed markedly. The activity decreased in the sequence Au/TiO2 (NaA) > Au/TiO2 (PNaA) > Au/TiO2 (none) > Au/TiO2 (PVA) > Au/TiO2 (PVP), despite the Au/TiO2 (none) catalyst possessing a larger Au mean particle size, suggesting active site blocking due to the steric nature of the polymer species. According to UV–vis, X-ray photoelectron spectroscopy (XPS), in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), high-resolution transmission electron microscopy (HRTEM), X-ray absorption spectroscopy (XAS), and composition analysis experiments, it was concluded that the enhanced activity of Au/TiO2 (NaA) and Au/TiO2 (PNaA) catalysts were attributed to the large proportions of low coordinate step/kink Au sites. It is to be noted that the electronic interactions between NaA and HAuCl4 facilitated the production of active Au nanoparticles. Our findings highlight that the physicochemical properties of the stabilizer can profoundly influence the reactivity of supported metal catalysts prepared by sol-immobilization. These observations highlight the influence that various stabilizers have on the morphology of supported metal nanoparticles and provides an explanation for the low activity of catalysts prepared using common forms such as PVA and PVP.
中文翻译:

稳定剂对Au/TiO2催化剂CO氧化性能的影响
稳定剂通常用于合成具有明确形态和尺寸分布的纳米晶体,使其成为研究多相催化剂结构-活性关系的理想工具。虽然有充分证据表明稳定剂可以影响纳米晶体的结构和尺寸,但很少强调这些物质的性质如何影响催化性能。在此,不同的聚合物(聚丙烯酸钠(PNaA)、聚(乙烯醇)(PVA)和聚(乙烯基吡咯烷酮)(PVP))和单体丙烯酸钠(NaA)被用作合成金纳米颗粒的稳定剂支持在 TiO 2 上. 无论使用何种稳定剂,所有催化剂中的平均金粒径都是可比的;然而,这些催化剂对 CO 氧化的活性显着不同。尽管 Au/TiO 2 (NaA) > Au/TiO 2 (PNaA) > Au/TiO 2 (无) > Au/TiO 2 (PVA) > Au/TiO 2 (PVP),但活性降低二氧化钛2(无)催化剂具有较大的 Au 平均粒径,表明由于聚合物物质的空间性质而导致活性位点封闭。根据紫外可见光、X 射线光电子能谱 (XPS)、原位漫反射红外傅立叶变换光谱 (DRIFTS)、高分辨率透射电子显微镜 (HRTEM)、X 射线吸收光谱 (XAS) 和成分分析实验得出结论,Au/TiO 2 (NaA) 和Au/TiO 2 (PNaA) 催化剂的活性增强归因于低配位台阶/扭结Au 位点的比例较大。需要注意的是 NaA 和 HAuCl 4之间的电子相互作用促进了活性金纳米粒子的产生。我们的研究结果强调,稳定剂的物理化学性质可以深刻影响通过溶胶固定制备的负载金属催化剂的反应性。这些观察结果突出了各种稳定剂对负载型金属纳米粒子形态的影响,并解释了使用常见形式(如 PVA 和 PVP)制备的催化剂活性低的原因。
更新日期:2021-09-17
中文翻译:

稳定剂对Au/TiO2催化剂CO氧化性能的影响
稳定剂通常用于合成具有明确形态和尺寸分布的纳米晶体,使其成为研究多相催化剂结构-活性关系的理想工具。虽然有充分证据表明稳定剂可以影响纳米晶体的结构和尺寸,但很少强调这些物质的性质如何影响催化性能。在此,不同的聚合物(聚丙烯酸钠(PNaA)、聚(乙烯醇)(PVA)和聚(乙烯基吡咯烷酮)(PVP))和单体丙烯酸钠(NaA)被用作合成金纳米颗粒的稳定剂支持在 TiO 2 上. 无论使用何种稳定剂,所有催化剂中的平均金粒径都是可比的;然而,这些催化剂对 CO 氧化的活性显着不同。尽管 Au/TiO 2 (NaA) > Au/TiO 2 (PNaA) > Au/TiO 2 (无) > Au/TiO 2 (PVA) > Au/TiO 2 (PVP),但活性降低二氧化钛2(无)催化剂具有较大的 Au 平均粒径,表明由于聚合物物质的空间性质而导致活性位点封闭。根据紫外可见光、X 射线光电子能谱 (XPS)、原位漫反射红外傅立叶变换光谱 (DRIFTS)、高分辨率透射电子显微镜 (HRTEM)、X 射线吸收光谱 (XAS) 和成分分析实验得出结论,Au/TiO 2 (NaA) 和Au/TiO 2 (PNaA) 催化剂的活性增强归因于低配位台阶/扭结Au 位点的比例较大。需要注意的是 NaA 和 HAuCl 4之间的电子相互作用促进了活性金纳米粒子的产生。我们的研究结果强调,稳定剂的物理化学性质可以深刻影响通过溶胶固定制备的负载金属催化剂的反应性。这些观察结果突出了各种稳定剂对负载型金属纳米粒子形态的影响,并解释了使用常见形式(如 PVA 和 PVP)制备的催化剂活性低的原因。









































 京公网安备 11010802027423号
京公网安备 11010802027423号