当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of the PET Degrading Enzyme PETase Studied with DFT/MM Molecular Dynamics Simulations
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c03700 Carola Jerves 1, 2 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Saulo da Silva 1, 2 , Pedro A. Fernandes 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-03 , DOI: 10.1021/acscatal.1c03700 Carola Jerves 1, 2 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Saulo da Silva 1, 2 , Pedro A. Fernandes 1
Affiliation
|
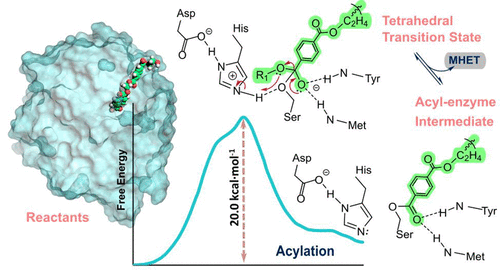
Polyethylene terephthalate (PET) has been widely used to make disposable bottles, among others, leading to massive PET waste accumulation in the environment. The discovery of the Ideonella sakaiensis PETase and MHETase enzymes, which hydrolyze PET into its constituting monomers, opened the possibility of a promising route for PET biorecycling. We describe an atomistic and thermodynamic interpretation of the catalytic reaction mechanism of PETase using umbrella sampling simulations at the robust PBE/MM MD level with a large QM region. The reaction mechanism takes place in two stages, acylation and deacylation, each of which occurs through a single, associative, concerted and asynchronous step. Acylation consists of proton transfer from Ser131 to His208, concerted with a nucleophilic attack by Ser131 on the substrate, leading to a tetrahedral transition state, which subsequently results in the release of MHET after the breaking of the ester bond. Deacylation is driven by deprotonation of an active site water molecule by His208, with the resulting hydroxide attacking the acylated Ser131 intermediate and breaking its bond to the substrate. Subsequently, His208 transfers the water proton to Ser131, with ensuant formation of MHET and enzyme regeneration. The rate-limiting acylation has a free energy barrier of 20.0 kcal·mol–1, consistent with the range of experimental values of 18.0–18.7 kcal·mol–1. Finally, we identify residues whose mutation should increase the enzyme turnover. Specifically, mutation of Asp83, Asp89, and Asp157 by nonpositive residues is expected to decrease the barrier of the rate-limiting step. This work led to the understanding of the catalytic mechanism of PETase and opened the way for additional rational enzyme engineering.
中文翻译:

用 DFT/MM 分子动力学模拟研究 PET 降解酶 PETase 的反应机理
聚对苯二甲酸乙二醇酯 (PET) 已被广泛用于制造一次性瓶子等,导致环境中大量 PET 废物积累。Ideonella sakaiensis的发现PETase 和 MHETase 酶将 PET 水解成其构成单体,为 PET 生物回收开辟了一条有前途的途径。我们在具有大 QM 区域的稳健 PBE/MM MD 水平上使用伞形采样模拟描述了对 PETase 催化反应机制的原子学和热力学解释。反应机理发生在两个阶段,即酰化和脱酰,每个阶段都通过一个单一的、关联的、协同的和异步的步骤发生。酰化包括从 Ser131 到 His208 的质子转移,伴随 Ser131 对底物的亲核攻击,导致四面体过渡态,随后导致酯键断裂后 MHET 的释放。脱酰基作用是由 His208 对活性位点水分子的去质子化驱动的,产生的氢氧化物攻击酰化的 Ser131 中间体并破坏其与底物的键。随后,His208 将水质子转移到 Ser131,随之形成 MHET 和酶再生。限速酰化的自由能垒为20.0 kcal·mol–1,与18.0–18.7 kcal·mol –1的实验值范围一致。最后,我们确定了突变会增加酶周转率的残基。具体而言,非阳性残基对 Asp83、Asp89 和 Asp157 的突变有望降低限速步骤的障碍。这项工作使人们了解了 PETase 的催化机制,并为其他合理的酶工程开辟了道路。
更新日期:2021-09-17
中文翻译:

用 DFT/MM 分子动力学模拟研究 PET 降解酶 PETase 的反应机理
聚对苯二甲酸乙二醇酯 (PET) 已被广泛用于制造一次性瓶子等,导致环境中大量 PET 废物积累。Ideonella sakaiensis的发现PETase 和 MHETase 酶将 PET 水解成其构成单体,为 PET 生物回收开辟了一条有前途的途径。我们在具有大 QM 区域的稳健 PBE/MM MD 水平上使用伞形采样模拟描述了对 PETase 催化反应机制的原子学和热力学解释。反应机理发生在两个阶段,即酰化和脱酰,每个阶段都通过一个单一的、关联的、协同的和异步的步骤发生。酰化包括从 Ser131 到 His208 的质子转移,伴随 Ser131 对底物的亲核攻击,导致四面体过渡态,随后导致酯键断裂后 MHET 的释放。脱酰基作用是由 His208 对活性位点水分子的去质子化驱动的,产生的氢氧化物攻击酰化的 Ser131 中间体并破坏其与底物的键。随后,His208 将水质子转移到 Ser131,随之形成 MHET 和酶再生。限速酰化的自由能垒为20.0 kcal·mol–1,与18.0–18.7 kcal·mol –1的实验值范围一致。最后,我们确定了突变会增加酶周转率的残基。具体而言,非阳性残基对 Asp83、Asp89 和 Asp157 的突变有望降低限速步骤的障碍。这项工作使人们了解了 PETase 的催化机制,并为其他合理的酶工程开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号